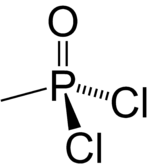
Methylphosphonyl dichloride (DC) or dichloro is an organophosphorus compound. It has commercial application in oligonucleotide synthesis,[1] but is most notable as being a precursor to several chemical weapons agents. It is a white crystalline solid that melts slightly above room temperature.[2]
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Methylphosphonic dichloride | |
| Other names
Methanephosphonic dichloride
Methanephosphonic acid dichloride Methylphosphonyl dichloride Dichloro | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.010.578 |
| EC Number |
|
PubChem CID
|
|
| UN number | 9206 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH3Cl2OP | |
| Molar mass | 132.91 g·mol−1 |
| Appearance | White crystalline solid |
| Density | 1.468 g/mL at 20 °C |
| Melting point | 28 to 34 °C (82 to 93 °F; 301 to 307 K) |
| Boiling point | 163 °C (325 °F; 436 K) |
| Reacts with water | |
| Solubility | Ether, THF |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Very toxic, reacts with water |
| GHS labelling: | |
 
| |
| Danger | |
| H314, H330 | |
| P260, P264, P271, P280, P284, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P320, P321, P363, P403+P233, P405, P501 | |
| Flash point | >110 °C |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
26 ppm/4h by inhalation (rat) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis and reactions
editMethylphosphonyl dichloride is produced by oxidation of methyldichlorophosphine, with sulfuryl chloride:[3]
- CH3PCl2 + SO2Cl2 → CH3P(O)Cl2 + SOCl2
It can also be produced from a range of methylphosphonates (e.g. dimethyl methylphosphonate) via chlorination with thionyl chloride. Various amines catalyse this process.[4] With hydrogen fluoride or sodium fluoride, it can be used to produce methylphosphonyl difluoride. With alcohols, it converts to the dialkoxide:[5]
- CH3P(O)Cl2 + 2 HOR → CH3P(O)(OR)2 + HCl
Safety
editMethylphosphonyl dichloride is very toxic and reacts vigorously with water to release hydrochloric acid. It is also listed under Schedule 2 of the Chemical Weapons Convention as it is used in the production of organophosphorus nerve agents such as sarin and soman.
References
edit- ^ Aldrich Handbook & Catalog of Fine Chemicals. Milwaukee, WI: Aldrich Chemical Company. 1994. p. 871. As cited in HSDB.
- ^ "SAFETY DATA SHEET Methylphosphonic dichloride". SAFETY DATA SHEET Methylphosphonic dichloride. MilliporeSigma. June 26, 2020. Retrieved April 27, 2022.
- ^ Svara, J.; Weferling, N.; Hofmann, T. "Phosphorus Compounds, Organic," In 'Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2008. doi:10.1002/14356007.a19_545.pub2.
- ^ Maier, Ludwig (1990). "Organic Phosphorus Compounds 90.l A Convenient, One-Step Synthesis of Alkyl- and Arylphosphonyl Dichlorides". Phosphorus, Sulfur, and Silicon and the Related Elements. 47 (3–4): 465–470. doi:10.1080/10426509008038002.
- ^ Carl Patois, Philippe Savignac, Elie About-Jaudet, Noël Collignon (1996). "Bis(Trifluoroethyl) (carboethoxymethyl)phosphonate". Organic Syntheses. 73: 152. doi:10.15227/orgsyn.073.0152.
{{cite journal}}: CS1 maint: multiple names: authors list (link)