SN2 reaction
This article uses too much jargon, which needs explaining or simplifying. (February 2024) |


The SN2 reaction (also known as bimolecular nucleophilic substitution) is a substitution reaction in organic chemistry. It is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it. This expels another group called a "leaving group". So, the incoming group replaces the leaving group in one step. Since two reacting species are involved in the slow, rate-determining step of the reaction, this leads to the name bimolecular nucleophilic substitution, or SN2. Among inorganic chemists, the SN2 reaction is often known as the interchange mechanism.
Reaction mechanism
[change | change source]The reaction most often occurs at an aliphatic sp3 carbon center with an electronegative, stable leaving group attached to it - 'X' - frequently a halide atom. The breaking of the C-X bond and the formation of the new C-Nu bond occur simultaneously to form a transition state in which the carbon under nucleophilic attack is pentacoordinate, and approximately sp2 hybridized. The nucleophile attacks the carbon at 180° to the leaving group, since this provides the best overlap between the nucleophile's lone pair and the C-X σ* antibonding orbital. The leaving group is then pushed off the opposite side and the product is formed.
If the substrate under nucleophilic attack is chiral, this can lead, although not necessarily, to an inversion of stereochemistry, called the Walden inversion.
In an example of the SN2 reaction, the attack of OH− (the nucleophile) on a bromoethane (the electrophile) results in ethanol, with bromide ejected as the leaving group:

A SN2 reaction occurs if the backside route of attack is not blocked by other atoms in the molecule (sterically hindered by substituents on the substrate). So, this mechanism usually occurs at an unhindered primary carbon center. If there is steric crowding on the substrate near the leaving group, such as at a tertiary carbon center, the substitution will use an SN1 rather than an SN2 mechanism, (an SN1 would also be more likely with blocked molecules because a sufficiently stable carbocation intermediary could be formed.)
In coordination chemistry, associative substitution proceeds by a similar mechanism as SN2.
Factors affecting the rate of the reaction
[change | change source]Four factors affect the rate of the reaction:
- Substrate. The substrate plays the most important part in determining the rate of the reaction. This is because the nucleophile attacks from the back of the substrate, thus breaking the carbon-leaving group bond and forming the carbon-nucleophile bond. Therefore, to maximise the rate of the SN2 reaction, the back of the substrate must be as unhindered as possible. Overall, this means that methyl and primary substrates react the fastest, followed by secondary substrates. Tertiary substrates do not participate in SN2 reactions, because of steric hindrance.
- Nucleophile. Like the substrate, steric hindrance affects the nucleophile's strength. The methoxide anion, for example, is both a strong base and nucleophile because it is a methyl nucleophile, and is thus very much unhindered. Tert-butoxide, on the other hand, is a strong base, but a poor nucleophile, because of its three methyl groups hindering its approach to the carbon. Nucleophile strength is also affected by charge and electronegativity: nucleophilicity increases with increasing negative charge and decreasing electronegativity. For example, OH- is a better nucleophile than water, and I- is a better nucleophile than Br- (in polar protic solvents). In a polar aprotic solvent, nucleophilicity increases up a column of the periodic table as there is no hydrogen bonding between the solvent and nucleophile. In this case, nucleophilicity mirrors basicity. I- would therefore be a weaker nucleophile than Br- because it is a weaker base.
- Solvent. The solvent affects the rate of reaction because solvents may or may not surround a nucleophile, thus hindering or not hindering its approach to the carbon atom. Polar aprotic solvents, like tetrahydrofuran, are better solvents for this reaction than polar protic solvents because polar protic solvents will be solvated by the solvent hydrogen bonding to the nucleophile. This hinders it from attacking the carbon with the leaving group.
- Leaving group. The leaving group affects the rate of reaction. The more stable leaving group is, the more likely that it will take the two electrons of its carbon-leaving group bond with it when the nucleophile attacks the carbon. Therefore, the weaker the leaving group is as a conjugate base, the better the leaving group. Similarly, the stronger its corresponding acid, the better the leaving group. Examples of good leaving groups are the halides (except fluoride) and tosylate. But HO- and H2N- are not good leaving groups.
Reaction kinetics
[change | change source]The rate of an SN2 reaction is second order, as the rate-determining step depends on the nucleophile concentration, [Nu−] as well as the concentration of substrate, [RX].
- r = k[RX][Nu−]
This is a key difference between the SN1 and SN2 mechanisms. In the SN1 reaction, the nucleophile attacks after the rate-limiting step is over. But in a SN2 reaction, the nucleophile forces off the leaving group in the limiting step. In other words, the rate of SN1 reactions depend only on the concentration of the substrate while the SN2 reaction rate depends on the concentration of both the substrate and nucleophile. In cases where both mechanisms are possible (for example at a secondary carbon center), the mechanism depends on solvent, temperature, concentration of the nucleophile or on the leaving group.
SN2 reactions are generally favored in primary alkyl halides or secondary alkyl halides with an aprotic solvent. They occur at a negligible rate in tertiary alkyl halides due to steric hindrance.
SN2 and SN1 are two extremes of a sliding scale of reactions. It is possible to find many reactions which exhibit both SN2 and SN1 character in their mechanisms. For instance, it is possible to get a contact ion pairs formed from an alkyl halide in which the ions are not fully separated. When these undergo substitution the stereochemistry will be inverted (as in SN2) for many of the reacting molecules but a few may show retention of configuration. SN2 reactions are more common than SN1 reactions.
E2 competition
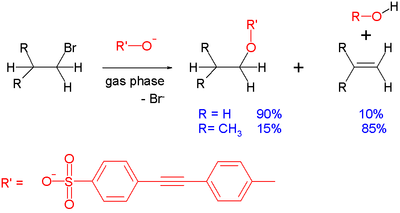
[change | change source]A common side reaction taking place with SN2 reactions is E2 elimination: the incoming anion can act as a base rather than as a nucleophile, removing a proton and leading to formation of the alkene. This effect can be demonstrated in the gas-phase reaction between a sulfonate and a simple alkyl bromide taking place inside a mass spectrometer:[1][2]
With ethyl bromide, the reaction product is predominantly the substitution product. As steric hindrance around the electrophilic center increases, as with isobutyl bromide, substitution is disfavored and elimination is the predominant reaction. Other factors favoring elimination are the strength of the base. With the less basic benzoate substrate, isopropyl bromide reacts with 55% substitution. In general, gas phase reactions and solution phase reactions of this type follow the same trends, even though in the first, solvent effects are dropped.
Roundabout mechanism
[change | change source]A development attracting attention in 2008 concerns a SN2 roundabout mechanism observed in a gas-phase reaction between chloride ions and methyl iodide with a special technique called crossed molecular beam imaging. When the chloride ions have sufficient velocity, the energy of the resulting iodide ions after the collision is much lower than expected, and it is theorized that energy is lost as a result of a full roundabout of the methyl group around the iodine atom before the actual displacement takes place.[3][4][5]
Related pages
[change | change source]References
[change | change source]- ↑ Gas Phase Studies of the Competition between Substitution and Elimination Reactions Scott Gronert Acc. Chem. Res.; 2003; 36(11) pp 848 - 857; (Article) doi:10.1021/ar020042n
- ↑ The technique used is electrospray ionization and because it requires charged reaction products for detection the nucleophile is fitted with an additional sulfonate anionic group, non-reactive and well separated from the other anion. The product ratio of substitution and elimination product can be measured from the intensity their relative molecular ions
- ↑ Imaging Nucleophilic Substitution Dynamics J. Mikosch, S. Trippel, C. Eichhorn, R. Otto, U. Lourderaj, J. X. Zhang, W. L. Hase, M. Weidemüller, and R. Wester Science 11 January 2008 319: 183-186 doi: 10.1126/science.1150238 (in Reports)
- ↑ PERSPECTIVES CHEMISTRY: Not So Simple John I. Brauman (11 January 2008) Science 319 (5860), 168. doi:10.1126/science.1152387
- ↑ Surprise From SN2 Snapshots Ion velocity measurements unveil additional unforeseen mechanism Carmen Drahl Chemical & Engineering News January 14, 2008 Volume 86, Number 2 p. 9 http://pubs.acs.org/cen/news/86/i02/8602notw1.html , video included