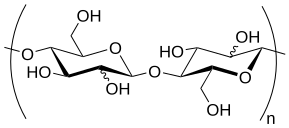
Glucomannan is a water-soluble polysaccharide that is considered a dietary fiber. It is a hemicellulose component in the cell walls of some plant species. Glucomannan is a food additive used as an emulsifier and thickener. It is a major source of mannan oligosaccharide (MOS) found in nature, the other being galactomannan, which is insoluble.[1]
| |
| Names | |
|---|---|
| IUPAC name
β(1→4)-d-gluco-d-mannoglycan
| |
| Identifiers | |
| ChEBI | |
| KEGG | |
PubChem CID
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Products containing glucomannan, under a variety of brand names, are marketed as dietary supplements with claims they can relieve constipation and help lower cholesterol levels.[2][3] Since 2010 they are legally marketed in Europe as helping with weight loss for people who are overweight and eating a diet with restricted calories,[3] but as of 2020[update] there was no good evidence that glucomannan helped weight loss.[4][5] Glucomannan lowers LDL cholesterol by 10 percent.[6]
Supplements containing glucomannans pose a risk for choking and bowel obstruction if they are not taken with sufficient water.[7] Other adverse effects include diarrhea, belching, and bloating; in one study people taking glucomannans had higher triglyceride levels.[8]
Glucomannans are also used to supplement animal feed for farmed animals, to cause the animals gain weight more quickly.
Chemistry
editGlucomannan is mainly a straight-chain polymer, with a small amount of branching. The component sugars are β-(1→4)-linked D-mannose and D-glucose in a ratio of 1.6:1.[9] The degree of branching is about 8% through β-(1→6)-glucosyl linkages.[10]
Glucomannan with α-(1→6)-linked galactose units in side branches is called galactoglucomannan.[citation needed]
Biological function
editIn the yeast cell wall, mannan oligosaccharides are present in complex molecules that are linked to the protein moiety. There are two main locations of mannan oligosaccharides in the surface area of Saccharomyces cerevisiae cell wall.[11]
They can be attached to the cell wall proteins as part of –O and –N glycosyl groups and also constitute elements of large α-D-mannanose polysaccharides (α-D-Mannans), which are built of α-(1,2)- and α-(1,3)- D-mannose branches (from 1 to 5 rings long), which are attached to long α-(1,6)-D-mannose chains.[12][13]
This specific combination of various functionalities involves mannan oligosaccharides-protein conjugates and highly hydrophilic and structurally variable 'brush-like' mannan oligosaccharides structures that can fit to various receptors of animal digestive tracts,[14] and to the receptors on the surface of bacterial membranes,[15] impacts these molecules' bioactivity. Mannan oligosaccharides-protein conjugates are involved in interactions with the animal's immune system and as result enhance immune system activity.[16] They also play a role in animal antioxidant and antimutagenic defense.[17]
Natural sources
editGlucomannan comprises 40% by dry weight of the roots, or corm, of the konjac plant. Another culinary source is salep, ground from the roots of certain orchids and used in Greek and Turkish cuisine. However, these orchid species are protected in the whole EU and the trade of salep is strictly forbidden. Glucomannan is also a hemicellulose that is present in large amounts in the wood of conifers and in smaller amounts in the wood of dicotyledons.[citation needed] Glucomannan is also a constituent of bacterial, plant and yeast cell wall with differences in the branches or glycosidic linkages in the linear structure.[18][19][20]
Uses
editHuman food additive
editGlucomannan is a food additive used as an emulsifier and thickener with the E number E425(ii).[21][22]
Glucomannan-rich salep powder is responsible for the unique textural properties of salep dondurma, a mastic-flavored stretchable and chewy ice cream of Turkish origin.[23]
Konjac, also rich in glucomannan, is widely used for its jelly-like texture. It found use in shirataki noodles, in fruit jellies snacks (with choking risk),[24] and as a substitute for gelatin.
Human dietary supplement
editGlucomannan is an ingredient in a variety of dietary supplement products marketed with claims that they aid in weight loss, but medical research has found no good evidence to support its use for this purpose.[4][5] The claim is that it makes a gel when mixed with water, which can take up space in the stomach and linger there longer than water alone would, inducing a person to feel full after having eaten a smaller amount of food.[8]
In Europe and Canada, glucomannan dietary supplements can be marketed with claims to lower cholesterol levels and to relieve constipation.[2][3]
Data from a randomized controlled clinical trial suggests that glucomannan dietary supplements help regulate the hormone ghrelin and might help control appetite in people with Type 2 diabetes.[25]
Health risks
editA health advisory was released by Health Canada stating the following: "Natural health products containing the ingredient glucomannan in tablet, capsule or powder form, which are currently on the Canadian market, have a potential for harm if taken without at least 250 ml or 8 ounces of water or other fluid. The risk includes choking and/or blockage of the throat, esophagus or intestine, according to international adverse reaction case reports. It is also important to note that these products should not be taken immediately before going to bed."[7]
Other adverse effects include diarrhea, belching, and bloating; in one study people taking glucomannans had higher triglyceride levels.[8]
Consumer issues
editSeveral companies have been determined by the Federal Trade Commission (FTC) or the Food and Drug Administration (FDA) to have, at some time, violated the Federal Food, Drug, and Cosmetic Act.[26] The companies include Vitacost,[27] PediaLean,[28] Herbal Worldwide Holdings,[29] BioTrim,[30] and others. The company Obesity Research Institute, the marketer of FiberThin, Zylotrim, Propolene and Lipozene, settled FTC charges that their misleading weight-loss claims violated federal laws by agreeing to pay $1.5 million in consumer redress.[31]
In 2001, a number of jelly-type candy products containing konjac-derived glucomannan were barred from import by the U.S. Food and Drug Administration due to choking hazards.[32]
Dietary supplements for animals
editIt is also used as dietary supplement for farmed animals in order to help them gain more weight from food, called the feed conversion ratio. The effect of mannan oligosaccharides on animal performance was analysed in meta-analyses for poultry,[33][34][35] pigs,[36][37] and calves.[38]
References
edit- ^ Nopvichai, C; Charoenwongpaiboon, T; Luengluepunya, N; Ito, K; Muanprasat, C; Pichyangkura, R (2019). "Production and purification of mannan oligosaccharide with epithelial tight junction enhancing activity". PeerJ. 7: e7206. doi:10.7717/peerj.7206. PMC 6611449. PMID 31304065.
MOS is often prepared by hydrolysis reaction of a mannose-contained glucan polymer, mainly glucomannan and galactomannan.
- ^ a b "Monograph: Glucomannan - Capsule". Health Canada. May 11, 2010.
- ^ a b c "Scientific Opinion on the substantiation of health claims related to konjac mannan (glucomannan) and reduction of body weight (ID 854, 1556, 3725), reduction of post-prandial glycaemic responses (ID 1559), maintenance of normal blood glucose concentration". EFSA Journal. 8 (10): 1798. October 2010. doi:10.2903/j.efsa.2010.1798.
- ^ a b Wharton S, Bonder R, Jeffery A, Christensen RA (2020). "The safety and effectiveness of commonly-marketed natural supplements for weight loss in populations with obesity: A critical review of the literature from 2006 to 2016". Crit Rev Food Sci Nutr (Systematic review). 60 (10): 1614–30. doi:10.1080/10408398.2019.1584873. PMID 30896252. S2CID 84843051.
GM appears generally safe, but there is insufficient evidence to suggest its efficacy in weight loss
- ^ a b Ríos-Hoyo A, Gutiérrez-Salmeán G (June 2016). "New Dietary Supplements for Obesity: What We Currently Know". Curr Obes Rep (Review). 5 (2): 262–70. doi:10.1007/s13679-016-0214-y. PMID 27053066. S2CID 12071766.
The more recent meta-analysis by Onakpoya et al. [58] revealed a non-statistically significant difference of −0.22 kg in weight loss between the glucomannan and placebo groups, contradicting the earlier meta-analysis. These conflicting results might be explained by the different inclusion criteria that the studies used to select the clinical trials analyzed. Although Sood et al. [59] found a statistically significant reduction in weight among study participants using glucomannan, this weight loss is not necessarily clinically significant; thus, the results should be interpreted carefully.
- ^ Ho, Hoang Vi Thanh; Jovanovski, Elena; Zurbau, Andreea; Blanco Mejia, Sonia; Sievenpiper, John L.; Au-Yeung, Fei; Jenkins, Alexandra L.; Duvnjak, Lea; Leiter, Lawrence; Vuksan, Vladimir (May 2017). "A systematic review and meta-analysis of randomized controlled trials of the effect of konjac glucomannan, a viscous soluble fiber, on LDL cholesterol and the new lipid targets non-HDL cholesterol and apolipoprotein B". The American Journal of Clinical Nutrition. 105 (5): 1239–1247. doi:10.3945/ajcn.116.142158. ISSN 1938-3207.
- ^ a b "Health Canada Advises Canadians that Natural Health Products containing Glucomannan May Cause Serious Choking if Used with Insufficient Fluid". Health Canada. January 29, 2010. Archived from the original on 4 February 2010.
- ^ a b c Onakpoya I, Posadzki P, Ernst E (2014). "The efficacy of glucomannan supplementation in overweight and obesity: a systematic review and meta-analysis of randomized clinical trials". J Am Coll Nutr. 33 (1): 70–78. doi:10.1080/07315724.2014.870013. PMID 24533610. S2CID 19874145.
- ^ Kaname Katsuraya; Kohsaku Okuyamab; Kenichi Hatanakab; Ryuichi Oshimab; Takaya Satoc; Kei Matsuzakic (2003). "Constitution of konjac glucomannan: chemical analysis and 13C NMR spectroscopy". Carbohydrate Polymers. 53 (2): 183–89. doi:10.1016/S0144-8617(03)00039-0.
- ^ "Glucomannan: Uses, Side Effects, Interactions and Warnings".
- ^ Stewart, GG; Russell, I (1998). "Brewer's Yeast". Brewing Science & Technology Series III.
- ^ Lesage, G.; Bussey, H. (2006). "Cell Wall Assembly in Saccharomyces cerevisiae". Microbiology and Molecular Biology Reviews. 70 (2): 317–43. doi:10.1128/MMBR.00038-05. PMC 1489534. PMID 16760306.
- ^ Vinogradov, E; Petersen, B; Bock, K (1998). "Discussion". Carbohydrate Research. 307 (1–2): 177–83. doi:10.1016/S0008-6215(98)00042-1. PMID 9658572.
- ^ Mansour, Michael K.; Levitz, Stuart M. (2003). "Fungal Mannoproteins: the Sweet Path to Immunodominance". ASM News. 69 (12): 595–600.
- ^ Garofalo, Corinne; Wellens, Adinda; Nguyen, Hien; Van Gerven, Nani; Slättegård, Rikard; Hernalsteens, Jean-Pierre; Wyns, Lode; Oscarson, Stefan; et al. (2008). Zhang, Shuguang (ed.). "Intervening with Urinary Tract Infections Using Anti-Adhesives Based on the Crystal Structure of the FimH–Oligomannose-3 Complex". PLOS ONE. 3 (4): e2040. Bibcode:2008PLoSO...3.2040W. doi:10.1371/journal.pone.0002040. PMC 2323111. PMID 18446213.
- ^ Wismar, René; Brix, Susanne; Frøkiaer, Hanne; Laerke, Helle Nygaard (2010). "Dietary fibers as immunoregulatory compounds in health and disease". Annals of the New York Academy of Sciences. 1190 (1): 70–85. Bibcode:2010NYASA1190...70W. doi:10.1111/j.1749-6632.2009.05256.x. PMID 20388138. S2CID 24236736.
- ^ Krizkova, L; Zitnanova, I; Mislovicova, D; Masarova, J; Sasinkova, V; Durackova, Z; Krajcovic, J (2006). "Antioxidant and antimutagenic activity of mannan neoglycoconjugates: Mannan–human serum albumine and mannan–penicillin G acylase". Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 606 (1–2): 72–79. doi:10.1016/j.mrgentox.2006.03.003. PMID 16677851.
- ^ Elbein, A. D. (1969)."Biosynthesis of a cell wall glucomannan in mung bean seedlings". Journal of Biological Chemistry, 244(6), 1608–16.
- ^ Tokoh, C., Takabe, K., Sugiyama, J., & Fujita, M. (2002). "CP/MAS 13C NMR and electron diffraction study of bacterial cellulose structure affected by cell wall polysaccharides". Cellulose, 9(3–4), 351–60.
- ^ Chorvatovičová, D., Machová, E., Šandula, J., & Kogan, G. (1999). "Protective effect of the yeast glucomannan against cyclophosphamide-induced mutagenicity". Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 444(1), 117–22.
- ^ Current EU approved additives and their E Numbers, Food Standards Agency, 26 November 2010
- ^ Mortensen, Alicja; Aguilar, Fernando; Crebelli, Riccardo; Di Domenico, Alessandro; Frutos, Maria Jose; Galtier, Pierre; Gott, David; Gundert-Remy, Ursula; Lambré, Claude; Leblanc, Jean-Charles; Lindtner, Oliver; Moldeus, Peter; Mosesso, Pasquale; Oskarsson, Agneta; Parent-Massin, Dominique; Stankovic, Ivan; Waalkens-Berendsen, Ine; Woutersen, Rudolf Antonius; Wright, Matthew; Younes, Maged; Brimer, Leon; Christodoulidou, Anna; Lodi, Federica; Tard, Alexandra; Dusemund, Birgit (June 2017). "Re-evaluation of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) as food additives". EFSA Journal. 15 (6): e04864. doi:10.2903/j.efsa.2017.4864. PMC 7009929. PMID 32625526.
- ^ "Ice Cream That's a Stretch", Harold McGee, The New York Times. 1 August 2007. Retrieved 27 September, 2018.
- ^ Draft Commission Decision (SANCO/362/2002) suspending the placing on the market and import of jelly confectionery containing food additive E 425 Konjac Archived 2011-04-05 at the Wayback Machine, UK Food Standards Agency, 8 March 2002
- ^ Chearskul, S.; Kriengsinyos, W.; Kooptiwut, S.; Sangurai, S.; Onreabroi, S.; Churintaraphan, M.; Semprasert, N.; Nitiyanant, W. (February 2009). "Immediate and long-term effects of glucomannan on total ghrelin and leptin in type 2 diabetes mellitus". Diabetes Research and Clinical Practice. 83 (2): e40–e42. doi:10.1016/j.diabres.2008.11.014. PMID 19108925.
- ^ "Ads for Various Diet Supplements and Topical Gels Don't Cut the Fat, Says the FTC". Federal Trade Commission
- ^ Food and Drug Administration letter
- ^ C. Landis Plummer (June 15, 2004). Docket No. 9318 Archived 2008-05-13 at the Wayback Machine. Federal Trade Commission.
- ^ Federal Trade Commission complaint
- ^ Kovacic, William E.; Chun, Barbara Y.K. (November 3, 2004). "Complaint for Permanent Injunction and Other Equitable Relief". United States District Court for the Central District of California.
- ^ "FTC Settles Claims with Marketers of FiberThin and Propolene". Federal Trade Commission. June 20, 2005
- ^ "Import Alert 33-15, Detention Without Physical Examination of Gel Candies Containing Konjac" US FDA, Retrieved April 1, 2016
- ^ Rosen, G. D. (2007). "Holo-analysis of the efficacy of Bio-Mos in broiler nutrition". British Poultry Science. 48 (1): 21–26. doi:10.1080/00071660601050755. PMID 17364536. S2CID 24138780.
- ^ Hooge, Danny M. (2004). "Turkey Pen Trials with Dietary Mannan Oligosaccharide: Meta-analysis, 1993-2003". 3 (3): 179–88. Archived from the original on 2012-03-22. Retrieved 2018-05-02.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Rosen, G. D. (2007). "Holo-analysis of the efficacy of Bio-Mos in turkey nutrition". British Poultry Science. 48 (1): 27–32. doi:10.1080/00071660601050730. PMID 17364537. S2CID 10865778.
- ^ Miguel, Jennifer C.; Rodriguez-Zas, Sandra L.; Pettigrew, James E. (2004). "Efficacy of a mannan oligosaccharide (Bio-Mos) for improving nursery pig performance". Journal of Swine Health and Production. 12 (6): 296–307.
- ^ Rosen, G. D. (2007). "Holo-analysis of the efficacy of Bio-Mos in pig nutrition". Animal Science. 82 (5): 683–89. doi:10.1079/ASC200684.
- ^ "Mannan Oligosaccharides: Natural Alternatives for Animal Nutrition (Part 3)" (PDF) (Press release). Milk Products. 2007. Archived from the original (PDF) on August 19, 2008.