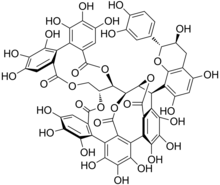
Acutissimin A
Appearance
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C56H38O31 | |
| Molar mass | 1206.88 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Acutissimin A is a flavono-ellagitannin, a type of tannin formed from the linking of a flavonoid with an ellagitannin.
In 2003, scientists at Institut Européen de Chimie et Biologie in Pessac, France found that when the oak tannin vescalagin interacts with a flavanoid in wine acutissimin A is created. In separate studies this phenolic compound has been shown to be 250 times more effective than the pharmaceutical drug Etoposide in stopping the growth of cancerous tumors.[1][2][3]
See also
[edit]References
[edit]- ^ Quideau S, Jourdes M, Saucier C, Glories Y, Pardon P, Baudry C (December 2003). "DNA topoisomerase inhibitor acutissimin a and other flavano-ellagitannins in red wine". Angewandte Chemie. 42 (48): 6012–4. doi:10.1002/anie.200352089. PMID 14679557.
- ^ Kashiwada Y, Nonaka G, Nishioka I, Chang JJ, Lee KH (August 1992). "Antitumor agents, 129. Tannins and related compounds as selective cytotoxic agents". Journal of Natural Products. 55 (8): 1033–43. doi:10.1021/np50086a002. PMID 1431932.
- ^ Quideau S, Jourdes M, Saucier C, Glories Y, Pardon P, Baudry C (2003). "DNA Topoisomerase Inhibitor Acutissimin A and Other Flavano-Ellagitannins in Red Wine". Angewandte Chemie. 115 (48): 6194–6. Bibcode:2003AngCh.115.6194Q. doi:10.1002/ange.200352089.
While it would be quite inappropriate to infer from the presence of acutissimin A in red wine that this beverage possesses antitumor properties, our work shows for the first time that wine contains polyphenolic molecules displaying both ellagitannin and flavanoid structural features.
