Scandium monosulfide
Appearance
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
| |
| Properties | |
| SSc | |
| Molar mass | 77.02 g·mol−1 |
| Appearance | gold-coloured solid[1] |
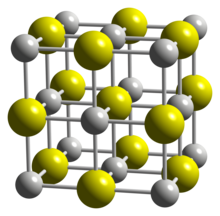
| Structure | |
| cubic | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Scandium monosulfide is a chemical compound of scandium and sulfur with the chemical formula ScS. Although its formula might suggest that it is a compound of scandium(II), i.e. [Sc2+][S2−], ScS is probably more realistically described as a pseudo-ionic compound, containing [Sc3+][S2−], with the remaining electron occupying the conduction band of the solid.[1]
Structure
[edit]Scandium monosulfide adopts the sodium chloride crystal structure type.[1]
Synthesis
[edit]Scandium monosulfide can be prepared by heating a mixture of scandium metal and powdered sulfur in the absence of air to 1150 °C for 70 hours.[1]
- Sc + S → ScS
References
[edit]- ^ a b c d Dismukes, J. P.; White, J. G. (1964). "The Preparation, Properties, and Crystal Structures of Some Scandium Sulfides in the Range Sc2S3-ScS". Inorg. Chem. 3 (9): 1220–1228. doi:10.1021/ic50019a004.
