Transition metal phosphate complex

Transition metal phosphate complexes are coordination complexes with one or more phosphate ligands. Phosphate binds to metals through one, two, three, or all four oxygen atoms. The bidentate coordination mode is common. The second and third pKa's of phosphoric acid, pKa2 and pKa3, are 7.2 and 12.37, respectively. It follows that HPO2−4 and PO3−4 are sufficiently basic to serve as ligands. The examples below confirm this expectation. Molecular metal phosphate complexes have no or few applications.
Examples
[edit]-
Co(H2NCH2CH2NH2)2PO4
-
[Pt2(HPO4)4(SC4H8)2]2−
-
[Re3Cl9(PO4)]3−
-
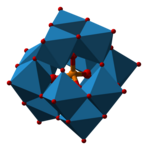
Phosphotungstic acid H3PW12O40
- Bidentate, chelating: One example is [Co(ethylenediamine)2(PO4)].[1]
- Bidentate, bridging: Phosphate, like carboxylate and sulfate, is well suited to span metal-metal bonds. This bonding mode is illustrated by [Mo2(HPO4)4]4−, which features a Mo-Mo triple bond.[2] Related [Pt(III)]2 complexes have been reported.[3]
- Tridentate, bridging. Several triangulo clusters feature a capping phosphate ligand, e.g. [Re3Cl9(PO4)]3−.[4]
- Encapsulated: In phosphotungstic acid, all four oxygen atoms of phosphate are bonded to metals.[5]
Other transition metal phosphates
[edit]Aside from molecular metal phosphate complexes, the topic of this article, many or most transition metal phosphates are nonmolecular, being coordination polymers or dense ternary or quaternary phases. Iron(III) phosphate, contemplated as a cathode material for batteries, is one example. Vanadyl phosphate (VOPO4(H2O)) is a commercial catalyst for oxidation reactions. Many metal phosphates occur as minerals.
Di- and polyphosphates
[edit]Phosphates exist in many condensed oligomeric forms. Many of these derivatives function as ligands for metal ions. Pyrophosphate (P2O4−7)[6] and trimetaphosphate ([P3O9]3−) have been particularly studied. They typically function as bi- and tridentate ligands.

References
[edit]- ^ Anderson, Bryan; Milburn, Ronald M.; Harrowfield, John M.; Robertson, Glen B.; Sargeson, Alan M. (1977). "Cobalt(III)-Promoted Hydrolysis of a Phosphate Ester". Journal of the American Chemical Society. 99 (8): 2652–2661. doi:10.1021/ja00450a042. PMID 850030.
- ^ Bino, Avi; Cotton, F. Albert (1979). "The Tetrakis(hydrogen Phosphato)dimolybdenum Ion, [Mo2(HPO4)4]2-. Compounds with a Metal-Metal Triple Bond Which Are Easily Prepared and Permanently Stable in Air". Inorganic Chemistry. 18 (12): 3562–3565. doi:10.1021/ic50202a053.
- ^ El-Mehdawi, Ramadan; Fronczek, Frank R.; Roundhill, D. Max (1986). "Axial Ligand Replacement Reactions in Tetrakis(mu-Phosphato)diplatinum(III) Complexes: Coordination of Amine, Thioether and Thiolate Functionalities". Inorganic Chemistry. 25 (8): 1155–1159. doi:10.1021/ic00228a019.
- ^ Irmler, Manfred; Meyer, Gerd (1990). "Sulfat, Phosphat und Arsenat als dreizähnige Liganden. Synthese und Kristallstrukturen von (NMe4)2[Re3Cl9O3SO], (NMe4)2(H7O3)[Re3Cl9O3PO] und (NMe4)2(H5O2)[Re3Cl9O3AsO]". Zeitschrift für Anorganische und Allgemeine Chemie. 587: 197–207. doi:10.1002/zaac.19905870121.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 1016. ISBN 978-0-08-037941-8.
- ^ Selmi, Ahmed; Akriche, Samah; Rzaigui, Mohamed (2009). "Bis(2-methylanilinium) diaquabis[dihydrogendiphosphato(2−)]cobaltate(II)". Acta Crystallographica Section E: Structure Reports Online. 65 (11): m1487. Bibcode:2009AcCrE..65M1487S. doi:10.1107/S1600536809044079. PMC 2971134. PMID 21578209.
- ^ S.Kamimura, T.Iida, K.Kanao, C.Nogawa, Y.Tanabe, K.Oh-ishi, S.Fukuzawa, Y.Ishii (2005). Journal of the Institute of Science and Engineering,Chuo University. 11: 1.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link)


![[Pt2(HPO4)4(SC4H8)2]2−](http://178.128.105.246/cars-http-upload.wikimedia.org/wikipedia/commons/thumb/0/07/CSD_CIF_DOXHAZ.png/106px-CSD_CIF_DOXHAZ.png)
![[Re3Cl9(PO4)]3−](http://178.128.105.246/cars-http-upload.wikimedia.org/wikipedia/commons/thumb/1/1c/CSD_CIF_KITKON01.png/148px-CSD_CIF_KITKON01.png)