User:Ramos1990/sandbox
| Home | Talk | Contributions | Images | Subpages | Todo | Toolbox | Sandbox | Userboxes |
Ping other users script - @Ramosxyjklkjl:
Wikipedia:Twinkle/doc for details on the meaning of twinkle functions.
[1] Request for Comments link (RFC)
Code for an archive bot. Copy and paste the code and adjust name on "archive=Talk:skskjdksjdksdj/Archive %(counter)d" line with "Talk:name of article"
|
no archives yet (create) |
{{Archives|auto=yes|search=yes}}
{{User:MiszaBot/config
| algo=old(90d)
| archive=Talk:nameofarticle/Archive %(counter)d
| counter=1
| maxarchivesize=150K
| archiveheader={{talkarchivenav|noredlinks=y}}
| minthreadsleft=5
| minthreadstoarchive=2
}}
Noncoding DNA successfully archived [2]. Myth page was successfully archived [3] see the code there as an example that worked.
Archive talk pages - bots WP:AUTOARCHIVE see [4]
How to move a page [5]
Closing a discussion template [6]
Various levels for page protection: [7]
An example of a successful page protection on April 10, 2017 [8]
"Category: Persecution by..." pages [9]
Useful Links :
WP:PAGEVIEW to see statistics on each wikipedia page such as views per day/month, how many editors are watching, etc
[10] How to use sfn notes and how to add quotes
2 Quote box examples form one page I saw one was detailed, the other very basic:
Talk Page guidelines and formatting [11]
[12] NPOV Policy "Acheiving Neutrality" says "As a general rule, do not remove sourced information from the encyclopedia solely on the grounds that it seems biased. Instead, try to rewrite the passage or section to achieve a more neutral tone. Biased information can usually be balanced with material cited to other sources to produce a more neutral perspective, so such problems should be fixed when possible through the normal editing process. Remove material only where you have a good reason to believe it misinforms or misleads readers in ways that cannot be addressed by rewriting the passage. The sections below offer specific guidance on common problems."
[13] "Gaming the system means deliberately using Wikipedia policies in bad faith to thwart the aims of Wikipedia. Gaming the system may represent an abuse of process, disruptive editing, or otherwise evading the spirit of community consensus. Editors typically game the system to make a point, to further an edit war, or to enforce a specific non-neutral point of view."
[14] for blockquotes or quote boxes
[15] for tags for head of article
[16] for wikipedia rules on synthesis WP:SYN and original research WP:OR
[17] tagging sentences with things like "citation needed", "page needed" and also template message boxes like "Section or article neutrality is disputed"
[18] on plagiarism and verifiability
[19] on Archiving Talk pages
inline citation wikipedia templates - inline templates i.e. page needed, citation needed, etc
WP:WINARS wikipedia is not a reliable source and how complex it is to fix everything on any article
WP:MEATPUPPET when editor recruits likeminded people to influence a page
WP:EXPERT on how wikipedia works and info on subject matter experts (SME)
WP:FORUMSHOP when editor recruits likeminded people to influence a page
WP:CANVASS when editor recruits likeminded people to influence a page
Deleted wikipedia article discussion - "Unscientific claims in religious scriptures" [20]
- of Page Views on any article [21]
[22] - font color template
- GREEN TEXT
- HIGHLIGHT YELLOW BACKGROUND
- white text with black background
All the text for the quote went here. Blah blah blah blah blah blah blah blah blah blah blah.
This is the source name goes Flavius Josephus: Antiquities of the Jews, Book 18, Chapter 3, 3[1] For Greek text see [23]
This is a very basic example I saw....Seems to align to the right automatically
User:Petergstrom topic ban thread on both medicine and religion topics due to aggressive behavior.[24]
Petergstrom edit war that resulted in Religiosity and intelligence page being protected.[25]
| Part of a series on |
| Chemistry |
|---|
 |
The following outline is provided as an overview of and topical guide to chemistry:
Chemistry is the science of atomic matter (matter that is composed of chemical elements), especially its chemical reactions, but also including its properties, structure, composition, behavior, and changes as they relate to the chemical reactions.[2][3] Chemistry is centrally concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds.
Summary of chemistry
[edit]Chemistry can be described as all of the following:
- An academic discipline – one with academic departments, curricula and degrees; national and international societies; and specialized journals.
- A scientific field (a branch of science) – widely recognized category of specialized expertise within science, and typically embodies its own terminology and nomenclature. Such a field will usually be represented by one or more scientific journals, where peer reviewed research is published. There are several chemistry-related scientific journals.
- A natural science – one that seeks to elucidate the rules that govern the natural world using empirical and scientific method.
- A physical science – one that studies non-living systems.
- A biological science – one that studies the role of chemicals and chemical processes in living organisms. See Outline of biochemistry.
- A natural science – one that seeks to elucidate the rules that govern the natural world using empirical and scientific method.
Branches of chemistry
[edit]- Physical chemistry – study of the physical and fundamental basis of chemical systems and processes. In particular, the energetics and dynamics of such systems and processes are of interest to physical chemists. Important areas of study include chemical thermodynamics, chemical kinetics, electrochemistry, statistical mechanics, spectroscopy, and more recently, astrochemistry.[4] Physical chemistry has large overlap with molecular physics. Physical chemistry involves the use of infinitesimal calculus in deriving equations. It is usually associated with quantum chemistry and theoretical chemistry. Physical chemistry is a distinct discipline from chemical physics, but again, there is very strong overlap.
- Chemical kinetics – study of rates of chemical processes.
- Chemical physics – investigates physicochemical phenomena using techniques from atomic and molecular physics and condensed matter physics; it is the branch of physics that studies chemical processes.
- Electrochemistry – branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor (the electrode: a metal or a semiconductor) and an ionic conductor (the electrolyte), and which involve electron transfer between the electrode and the electrolyte or species in solution.
- Femtochemistry – area of physical chemistry that studies chemical reactions on extremely short timescales, approximately 10−15 seconds (one femtosecond).
- Geochemistry – chemical study of the mechanisms behind major systems studied in geology.
- Photochemistry – study of chemical reactions that proceed with the absorption of light by atoms or molecules.
- Quantum chemistry – branch of chemistry whose primary focus is the application of quantum mechanics in physical models and experiments of chemical systems.
- Solid-state chemistry – study of the synthesis, structure, and properties of solid phase materials, particularly, but not necessarily exclusively of, non-molecular solids.
- Spectroscopy – study of the interaction between matter and radiated energy.
- Stereochemistry – study of the relative spatial arrangement of atoms that form the structure of molecules
- Surface science – study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid-gas interfaces.
- Thermochemistry –The branch of chemistry that studies the relation between chemical action and the amount of heat absorbed or generated.
- Calorimetry – The study of heat changes in physical and chemical processes.
- Organic chemistry (outline) – study of the structure, properties, composition, mechanisms, and reactions of organic compounds. An organic compound is defined as any compound based on a carbon skeleton.
- Biochemistry – study of the chemicals, chemical reactions and chemical interactions that take place in living organisms. Biochemistry and organic chemistry are closely related, as in medicinal chemistry or neurochemistry. Biochemistry is also associated with molecular biology and genetics.
- Neurochemistry – study of neurochemicals; including transmitters, peptides, proteins, lipids, sugars, and nucleic acids; their interactions, and the roles they play in forming, maintaining, and modifying the nervous system.
- Molecular biochemistry and genetic engineering –an area of biochemistry and molecular biology that studies the genes, their heritage and their expression.
- Bioorganic chemistry – combines organic chemistry and biochemistry toward biology.
- Biophysical chemistry – is a physical science that uses the concepts of physics and physical chemistry for the study of biological systems.
- Medicinal chemistry – discipline which applies chemistry for medical or drug related purposes.
- Organometallic chemistry – is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkaline, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and tin.
- Physical organic chemistry – study of the interrelationships between structure and reactivity in organic molecules.
- Polymer chemistry – multidisciplinary science that deals with the chemical synthesis and chemical properties of polymers or macromolecules.
- Click chemistry
- Biochemistry – study of the chemicals, chemical reactions and chemical interactions that take place in living organisms. Biochemistry and organic chemistry are closely related, as in medicinal chemistry or neurochemistry. Biochemistry is also associated with molecular biology and genetics.
- Inorganic chemistry – study of the properties and reactions of inorganic compounds. The distinction between organic and inorganic disciplines is not absolute and there is much overlap, most importantly in the sub-discipline of organometallic chemistry.
- Bioinorganic chemistry
- Cluster chemistry
- Materials chemistry – preparation, characterization, and understanding of substances with a useful function. The field is a new breadth of study in graduate programs, and it integrates elements from all classical areas of chemistry with a focus on fundamental issues that are unique to materials. Primary systems of study include the chemistry of condensed phases (solids, liquids, polymers) and interfaces between different phases.
- Nuclear chemistry – study of how subatomic particles come together and make nuclei. Modern Transmutation is a large component of nuclear chemistry, and the table of nuclides is an important result and tool for this field.
- Analytical chemistry – analysis of material samples to gain an understanding of their chemical composition and structure. Analytical chemistry incorporates standardized experimental methods in chemistry. These methods may be used in all subdisciplines of chemistry, excluding purely theoretical chemistry.
- Other
- Astrochemistry – study of the abundance and reactions of chemical elements and molecules in the universe, and their interaction with radiation.
- Cosmochemistry – study of the chemical composition of matter in the universe and the processes that led to those compositions.
- Computational chemistry
- Environmental chemistry – study of chemical and biochemical phenomena that occur diverse aspects of the environment such the air, soil, and water. It also studies the effects of human activity on the environment.
- Green chemistry is a philosophy of chemical research and engineering that encourages the design of products and processes that minimize the use and generation of hazardous substances.
- Supramolecular chemistry – refers to the domain of chemistry beyond that of molecules and focuses on the chemical systems made up of a discrete number of assembled molecular subunits or components.
- Theoretical chemistry – study of chemistry via fundamental theoretical reasoning (usually within mathematics or physics). In particular the application of quantum mechanics to chemistry is called quantum chemistry. Since the end of the Second World War, the development of computers has allowed a systematic development of computational chemistry, which is the art of developing and applying computer programs for solving chemical problems. Theoretical chemistry has large overlap with (theoretical and experimental) condensed matter physics and molecular physics.
- Wet chemistry
- Agrochemistry – study and application of both chemistry and biochemistry for agricultural production, the processing of raw products into foods and beverages, and environmental monitoring and remediation.
- Atmospheric chemistry – branch of atmospheric science which studies the chemistry of the Earth's atmosphere and that of other planets.
- Chemical engineering – branch of engineering that applies the physical sciences (e.g., chemistry and physics) and/or life sciences (e.g., biology, microbiology and biochemistry) together with mathematics and economics to processes that convert raw materials or chemicals into more useful or valuable forms.
- Chemical biology – scientific discipline spanning the fields of chemistry and biology and involves the application of chemical techniques and tools, often compounds produced through synthetic chemistry, to analyze and manipulation of biological systems.
- Chemo-informatics – use of computer and informational techniques applied to a range of problems in the field of chemistry.
- Flow chemistry – study of chemical reactions in continuous flow, not as stationary batches, in industry and macro processing equipment.
- Immunohistochemistry – involves the process of detecting antigens (e.g., proteins) in cells of a tissue section by exploiting the principle of antibodies binding specifically to antigens in biological tissues.
- Immunochemistry – is a branch of chemistry that involves the study of the reactions and components on the immune system.
- Chemical oceanography – study of ocean chemistry: the behavior of the chemical elements within the Earth's oceans
- Materials science – is an interdisciplinary field investigating the relationship between the structure of materials at atomic or molecular scales and their macroscopic properties.
- Mathematical chemistry – area of study engaged in novel applications of mathematics to chemistry. It concerns itself principally with the mathematical modeling of chemical phenomena.
- Mechanochemistry – coupling of mechanical and chemical phenomena on a molecular scale and can be seen as a coupling of chemistry and mechanical engineering.
- Molecular biology – study of interactions between the various systems of a cell. It overlaps with biochemistry.
- Molecular mechanics – applies classical mechanics to model molecular systems.
- Nanotechnology – study and application of matter that is at an atomic and molecular scale. This broad field interacts with chemistry at such scales.
- Petrochemistry – study of the transformation of petroleum and natural gas into useful products or raw materials.
- Pharmacology – branch of medicine and biology concerned with the study of drug action along with the chemical effects.
- Phytochemistry – study of phytochemicals which come from plants.
- Radiochemistry – chemistry of radioactive materials.
- Sonochemistry – study of effect of sonic waves and wave properties on chemical systems.
- Synthetic chemistry – study of chemical synthesis.
- Toxicology – study of the adverse effects of chemical substances on living organism and the practice of diagnosing and treating exposures to toxins and toxicants.
- Astrochemistry – study of the abundance and reactions of chemical elements and molecules in the universe, and their interaction with radiation.
History of chemistry
[edit]- Precursors to chemistry
- History of the branches of chemistry
- History of analytical chemistry – history of the study of the separation, identification, and quantification of the chemical components of natural and artificial materials.
- History of astrochemistry – history of the study of the abundance and reactions of chemical elements and molecules in the universe, and their interaction with radiation.
- History of cosmochemistry – history of the study of the chemical composition of matter in the universe and the processes that led to those compositions
- History of atmospheric chemistry – history of the branch of atmospheric science in which the chemistry of the Earth's atmosphere and that of other planets is studied. It is a multidisciplinary field of research and draws on environmental chemistry, physics, meteorology, computer modeling, oceanography, geology and volcanology and other disciplines
- History of biochemistry – history of the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes.
- History of agrochemistry – history of the study of both chemistry and biochemistry which are important in agricultural production, the processing of raw products into foods and beverages, and in environmental monitoring and remediation.
- History of bioinorganic chemistry – history of the examines the role of metals in biology.
- History of bioorganic chemistry – history of the rapidly growing scientific discipline that combines organic chemistry and biochemistry.
- History of biophysical chemistry – history of the new branch of chemistry that covers a broad spectrum of research activities involving biological systems.
- History of environmental chemistry – history of the scientific study of the chemical and biochemical phenomena that occur in natural places.
- History of immunochemistry – history of the branch of chemistry that involves the study of the reactions and components on the immune system.
- History of medicinal chemistry – history of the discipline at the intersection of chemistry, especially synthetic organic chemistry, and pharmacology and various other biological specialties, where they are involved with design, chemical synthesis and development for market of pharmaceutical agents (drugs).
- History of pharmacology – history of the branch of medicine and biology concerned with the study of drug action.
- History of natural product chemistry – history of the chemical compound or substance produced by a living organism – history of the found in nature that usually has a pharmacological or biological activity for use in pharmaceutical drug discovery and drug design.
- History of neurochemistry – history of the specific study of neurochemicals, which include neurotransmitters and other molecules such as neuro-active drugs that influence neuron function.
- History of computational chemistry – history of the branch of chemistry that uses principles of computer science to assist in solving chemical problems.
- History of chemo-informatics – history of the use of computer and informational techniques, applied to a range of problems in the field of chemistry.
- History of molecular mechanics – history of the uses Newtonian mechanics to model molecular systems.
- History of Flavor chemistry – history of the someone who uses chemistry to engineer artificial and natural flavors.
- History of Flow chemistry – history of the chemical reaction is run in a continuously flowing stream rather than in batch production.
- History of geochemistry – history of the study of the mechanisms behind major geological systems using chemistry
- History of aqueous geochemistry – history of the study of the role of various elements in watersheds, including copper, sulfur, mercury, and how elemental fluxes are exchanged through atmospheric-terrestrial-aquatic interactions
- History of isotope geochemistry – history of the study of the relative and absolute concentrations of the elements and their isotopes using chemistry and geology
- History of ocean chemistry – history of the studies the chemistry of marine environments including the influences of different variables.
- History of organic geochemistry – history of the study of the impacts and processes that organisms have had on Earth
- History of regional, environmental and exploration geochemistry – history of the study of the spatial variation in the chemical composition of materials at the surface of the Earth
- History of inorganic chemistry – history of the branch of chemistry concerned with the properties and behavior of inorganic compounds.
- History of nuclear chemistry – history of the subfield of chemistry dealing with radioactivity, nuclear processes and nuclear properties.
- History of radiochemistry – history of the chemistry of radioactive materials, where radioactive isotopes of elements are used to study the properties and chemical reactions of non-radioactive isotopes (often within radiochemistry the absence of radioactivity leads to a substance being described as being inactive as the isotopes are stable).
- History of organic chemistry – history of the study of the structure, properties, composition, reactions, and preparation (by synthesis or by other means) of carbon-based compounds, hydrocarbons, and their derivatives.
- History of petrochemistry – history of the branch of chemistry that studies the transformation of crude oil (petroleum) and natural gas into useful products or raw materials.
- History of organometallic chemistry – history of the study of chemical compounds containing bonds between carbon and a metal.
- History of photochemistry – history of the study of chemical reactions that proceed with the absorption of light by atoms or molecules..
- History of physical chemistry – history of the study of macroscopic, atomic, subatomic, and particulate phenomena in chemical systems in terms of physical laws and concepts.
- History of chemical kinetics – history of the study of rates of chemical processes.
- History of chemical thermodynamics – history of the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics.
- History of electrochemistry – history of the branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor (a metal or a semiconductor) and an ionic conductor (the electrolyte), and which involve electron transfer between the electrode and the electrolyte or species in solution.
- History of Femtochemistry – history of the Femtochemistry is the science that studies chemical reactions on extremely short timescales, approximately 10−15 seconds (one femtosecond, hence the name).
- History of mathematical chemistry – history of the area of research engaged in novel applications of mathematics to chemistry; it concerns itself principally with the mathematical modeling of chemical phenomena.
- History of mechanochemistry – history of the coupling of the mechanical and the chemical phenomena on a molecular scale and includes mechanical breakage, chemical behaviour of mechanically stressed solids (e.g., stress-corrosion cracking), tribology, polymer degradation under shear, cavitation-related phenomena (e.g., sonochemistry and sonoluminescence), shock wave chemistry and physics, and even the burgeoning field of molecular machines.
- History of physical organic chemistry – history of the study of the interrelationships between structure and reactivity in organic molecules.
- History of quantum chemistry – history of the branch of chemistry whose primary focus is the application of quantum mechanics in physical models and experiments of chemical systems.
- History of sonochemistry – history of the study of the effect of sonic waves and wave properties on chemical systems.
- History of stereochemistry – history of the study of the relative spatial arrangement of atoms within molecules.
- History of supramolecular chemistry – history of the area of chemistry beyond the molecules and focuses on the chemical systems made up of a discrete number of assembled molecular subunits or components.
- History of thermochemistry – history of the study of the energy and heat associated with chemical reactions and/or physical transformations.
- History of phytochemistry – history of the strict sense of the word the study of phytochemicals.
- History of polymer chemistry – history of the multidisciplinary science that deals with the chemical synthesis and chemical properties of polymers or macromolecules.
- History of solid-state chemistry – history of the study of the synthesis, structure, and properties of solid phase materials, particularly, but not necessarily exclusively of, non-molecular solids
- History of multidisciplinary fields involving chemistry:
- History of chemical biology – history of the scientific discipline spanning the fields of chemistry and biology that involves the application of chemical techniques and tools, often compounds produced through synthetic chemistry, to the study and manipulation of biological systems.
- History of chemical engineering – history of the branch of engineering that deals with physical science (e.g., chemistry and physics), and life sciences (e.g., biology, microbiology and biochemistry) with mathematics and economics, to the process of converting raw materials or chemicals into more useful or valuable forms.
- History of chemical oceanography – history of the study of the behavior of the chemical elements within the Earth's oceans.
- History of chemical physics – history of the branch of physics that studies chemical processes from the point of view of physics.
- History of materials science – history of the interdisciplinary field applying the properties of matter to various areas of science and engineering.
- History of nanotechnology – history of the study of manipulating matter on an atomic and molecular scale
- History of oenology – history of the science and study of all aspects of wine and winemaking except vine-growing and grape-harvesting, which is a subfield called viticulture.
- History of spectroscopy – history of the study of the interaction between matter and radiated energy
- History of surface science – history of the Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid–gas interfaces.
- History of chemicals
- History of chemical processes
- History of the chemical industry
- History of the periodic table
Chemicals
[edit]Atomic Theory
[edit]- Atomic models
- Atomism – Natural philosophy that theorizes that the world is composed of indivisible pieces.
- Plum pudding model
- Rutherford model
- Bohr model
Thermochemistry
[edit]Terminology
[edit]- Thermochemistry –
- Chemical kinetics – the study of the rates of chemical reactions and investigates how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that can describe the characteristics of a chemical reaction.
- Exothermic –a process or reaction in which the system release energy to its surroundings in the form of heat. They are denoted by negative heat flow.
- Endothermic –a process or reaction in which the system absorbs energy from its surroundings in the form of heat. They are denoted by positive heat flow.
- Thermochemical equation –
- Enthalpy change – internal energy of a system plus the product of pressure and volume. Its change in a system is equal to the heat brought to the system at constant pressure.
- Enthalpy of reaction –
- Temperature – an objective comparative measure of heat.
- Calorimeter – an object used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity.
- Heat – A form of energy associated with the kinetic energy of atoms or molecules and capable of being transmitted through solid and fluid media by conduction, through fluid media by convection, and through empty space by radiation.[5]
- Joule – a unit of energy.
- Calorie –
- Specific heat –
- Specific heat capacity –
- Latent heat –
- Heat of fusion –
- Heat of vaporization –
- Collision theory –
- Activation energy –
- Activated complex –
- Reaction rate –
- Catalyst –
Thermochemical Equations
[edit]- Chemical equations that include the heat involved in a reaction, either on the reactant side or the product side.
- Examples:
- H2O(l) + 240kJ → H2O(g)
- N2 + 3H2 → 2NH3 + 92kJ
- Joule (J) –
Enthalpy
[edit]How to calculate the enthalpy of N₂+3H₂ ⇌ 2NH₃?
Enthalpy and Thermochemical Equations
[edit]Endothermic Reactions
[edit]Exothermic Reactions
[edit]Potential Energy Diagrams
[edit]Thermochemistry Stoichiometry
[edit]Chemists
[edit]- For more chemists, see: Nobel Prize in Chemistry and List of chemists
- Elias James Corey
- Marie Curie
- John Dalton
- Humphry Davy
- Eleuthère Irénée du Pont
- George Eastman
- Michael Faraday
- Dmitriy Mendeleyev
- Alfred Nobel
- Wilhelm Ostwald
- Louis Pasteur
- Linus Pauling
- Joseph Priestley
- Karl Ziegler
- Ahmed Zewail
- Robert Burns Woodward
- Rosalind Franklin
- Amedeo Avogadro
Chemistry literature
[edit]- Scientific literature –
- Scientific journal –
- Academic journal –
- List of important publications in chemistry
- List of scientific journals in chemistry
Lists
[edit]- List of chemical elements — atomic mass, atomic number, symbol, name
- List of minerals - Minerals
- Electron configurations of the elements (data page) — electron configuration, electrons per shell
- Densities of the elements (data page) — density (solid, liquid, gas)
- Electron affinity (data page) — electron affinity
- Melting points of the elements (data page) — melting point
- Boiling points of the elements (data page) — boiling point
- Critical points of the elements (data page) — critical point
- Heats of fusion of the elements (data page) — heat of fusion
- Heats of vaporization of the elements (data page) — heat of vaporization
- Heat capacities of the elements (data page) — heat capacity
- Vapor pressures of the elements (data page) — vapor pressure
- Electronegativities of the elements (data page) — electronegativity (Pauling scale)
- Ionization energies of the elements (data page) — ionization energies (in eV) and molar ionization energies (in kJ/mol)
- Atomic radii of the elements (data page) — atomic radius (empirical), atomic radius (calculated), van der Waals radius, covalent radius
- Electrical resistivities of the elements (data page) — electrical resistivity
- Thermal conductivities of the elements (data page) — thermal conductivity
- Thermal expansion coefficients of the elements (data page) — thermal expansion
- Speeds of sound of the elements (data page) — speed of sound
- Elastic properties of the elements (data page) — Young's modulus, Poisson ratio, bulk modulus, shear modulus
- Hardnesses of the elements (data page) — Mohs hardness, Vickers hardness, Brinell hardness
- Abundances of the elements (data page) — Earth's crust, sea water, Sun and solar system
- List of oxidation states of the elements — oxidation states
- List of CAS numbers by chemical compound
- List of Extremely Hazardous Substances
- List of inorganic compounds
- List of organic compounds
- List of alkanes
- List of alloys
- Other
- List of thermal conductivities
- List of purification methods in chemistry
- List of unsolved problems in chemistry
See also
[edit]References
[edit]- ^ Flavius Josephus: Antiquities of the Jews, Book 18, Chapter 3, 3, based on the translation of Louis H. Feldman, The Loeb Classical Library. http://www.josephus.org/testimonium.htm
- ^ "What is Chemistry?". Chemweb.ucc.ie. Retrieved 2011-06-12.
- ^ Chemistry. (n.d.). Merriam-Webster's Medical Dictionary. Retrieved August 19, 2007.
- ^ Herbst, Eric (May 12, 2005). "Chemistry of Star-Forming Regions". Journal of Physical Chemistry A. 109 (18): 4017–4029. Bibcode:2005JPCA..109.4017H. doi:10.1021/jp050461c. PMID 16833724.
- ^ "heat", The Free Dictionary, retrieved 2019-08-18
External links
[edit]- International Union of Pure and Applied Chemistry
- IUPAC Nomenclature Home Page, see especially the "Gold Book" containing definitions of standard chemical terms
- Interactive Mind Map of Chemistry
- / Chemical energetics
Chemistry Chemistry [:[Category:Chemistry-related lists| ]]
Molecular motors are natural (biological) or artificial molecular machines that are the essential agents of movement in living organisms. In general terms, a motor is a device that consumes energy in one form and converts it into motion or mechanical work; for example, many protein-based molecular motors harness the chemical free energy released by the hydrolysis of ATP in order to perform mechanical work.[1] In terms of energetic efficiency, this type of motor can be superior to currently available man-made motors. One important difference between molecular motors and macroscopic motors is that molecular motors operate in the thermal bath, an environment in which the fluctuations due to thermal noise are significant.
Examples
[edit]
Some examples of biologically important molecular motors:[2]
- Cytoskeletal motors
- Myosins are responsible for muscle contraction, intracellular cargo transport, and producing cellular tension.
- Kinesin moves cargo inside cells away from the nucleus along microtubules, in anterograde transport.
- Dynein produces the axonemal beating of cilia and flagella and also transports cargo along microtubules towards the cell nucleus, in retrograde transport.
- Polymerisation motors
- Rotary motors:
- FoF1-ATP synthase family of proteins convert the chemical energy in ATP to the electrochemical potential energy of a proton gradient across a membrane or the other way around. The catalysis of the chemical reaction and the movement of protons are coupled to each other via the mechanical rotation of parts of the complex. This is involved in ATP synthesis in the mitochondria and chloroplasts as well as in pumping of protons across the vacuolar membrane.[3]
- The bacterial flagellum responsible for the swimming and tumbling of E. coli and other bacteria acts as a rigid propeller that is powered by a rotary motor. This motor is driven by the flow of protons across a membrane, possibly using a similar mechanism to that found in the Fo motor in ATP synthase.
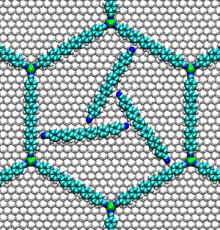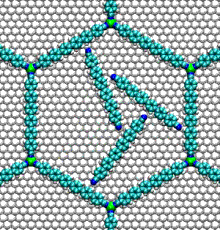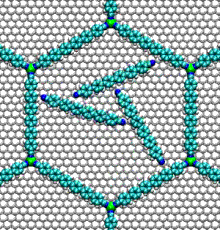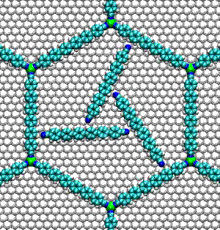
- Nucleic acid motors:
- RNA polymerase transcribes RNA from a DNA template.[5]
- DNA polymerase turns single-stranded DNA into double-stranded DNA.[6]
- Helicases separate double strands of nucleic acids prior to transcription or replication. ATP is used.
- Topoisomerases reduce supercoiling of DNA in the cell. ATP is used.
- RSC and SWI/SNF complexes remodel chromatin in eukaryotic cells. ATP is used.
- SMC proteins responsible for chromosome condensation in eukaryotic cells.[7]
- Viral DNA packaging motors inject viral genomic DNA into capsids as part of their replication cycle, packing it very tightly.[8] Several models have been put forward to explain how the protein generates the force required to drive the DNA into the capsid; for a review, see. [26] An alternative proposal is that, in contrast with all other biological motors, the force is not generated directly by the protein, but by the DNA itself.[9] In this model, ATP hydrolysis is used to drive protein conformational changes that alternatively dehydrate and rehydrate the DNA, cyclically driving it from B-DNA to A-DNA and back again. A-DNA is 23% shorter than B-DNA, and the DNA shrink/expand cycle is coupled to a protein-DNA grip/release cycle to generate the forward motion that propels DNA into the capsid.
- Enzymatic motors: The enzymes below have been shown to diffuse faster in the presence of their catalytic substrates, known as enhanced diffusion. They also have been shown to move directionally in a gradient of their substrates, known as chemotaxis. Their mechanisms of diffusion and chemotaxis are still debated. Possible mechanisms include solutal buoyancy, phoresis or conformational changes.[10][11][12]
- Catalase
- Urease
- Aldolase
- Hexokinase
- Phosphoglucose isomerase
- Phosphofructokinase
- Glucose Oxidase
A recent study has also shown that certain enzymes, such as Hexokinase and Glucose Oxidase, are aggregating or fragmenting during catalysis. This changes their hydrodynamic size that can affect enhanced diffusion measurements.[13]
- Synthetic molecular motors have been created by chemists that yield rotation, possibly generating torque.[14]
Organelle and vesicle transport
[edit]There are two major families of molecular motors that transport organelles throughout the cell. These families include the dynein family and the kinesin family. Both have very different structures from one another and different ways of achieving a similar goal of moving organelles around the cell. These distances, though only few micrometers, are all preplanned out using microtubules.[15]
- Kinesin - These molecular motors always move towards the positive end of the cell
- Uses ATP hydrolysis during the process converting ATP to ADP
- This process consists of . . .
- The "foot" of the motor binds using ATP, the "foot" proceeds a step, and then ADP comes off. This repeats itself until the destination has been reached
- This process consists of . . .
- The kinesin family consists of a multitude of different motor types
- Kinesin-1 (Conventional)
- Kinesin-2 (Heterotrimeric)
- Kinesin-5 (Bipolar)
- Kinesin-13
- Uses ATP hydrolysis during the process converting ATP to ADP
- Dynein - These molecular motors always move towards the negative end of the cell
- Uses ATP hydrolysis during the process converting ATP to ADP
- Unlike kinesin, the dynein is structured in a different way which requires it to have different movement methods.
- One of these methods includes the power stroke, which allows the motor protein to "crawl" along the microtubule to its location.
- The structure of Dynein consists of
- A Stem Containing
- A region that binds to dynactin
- Intermediate/light chains that will attach to the dynactin bonding region
- A Head
- A Stalk
- With a domain that will bind to the microtubule
These molecular motors tend to take the path of the microtubules. This is most likely due to the facts that the microtubules spring forth out of the centrosome and surround the entire volume of the cell. This in tern creates a "Rail system" of the whole cell and paths leading to its organelles.
- With a domain that will bind to the microtubule
- A Stem Containing
Theoretical considerations
[edit]| Part of a series on |
| Microbial and microbot movement |
|---|
 |
| Microswimmers |
| Molecular motors |
Because the motor events are stochastic, molecular motors are often modeled with the Fokker–Planck equation or with Monte Carlo methods. These theoretical models are especially useful when treating the molecular motor as a Brownian motor.
Experimental observation
[edit]In experimental biophysics, the activity of molecular motors is observed with many different experimental approaches, among them:
- Fluorescent methods: fluorescence resonance energy transfer (FRET), fluorescence correlation spectroscopy (FCS), total internal reflection fluorescence (TIRF).
- Magnetic tweezers can also be useful for analysis of motors that operate on long pieces of DNA.
- Neutron spin echo spectroscopy can be used to observe motion on nanosecond timescales.
- Optical tweezers (not to be confused with molecular tweezers in context) are well-suited for studying molecular motors because of their low spring constants.
- Scattering techniques: single particle tracking based on dark field microscopy or interferometric scattering microscopy (iSCAT)
- Single-molecule electrophysiology can be used to measure the dynamics of individual ion channels.
Many more techniques are also used. As new technologies and methods are developed, it is expected that knowledge of naturally occurring molecular motors will be helpful in constructing synthetic nanoscale motors.
Non-biological
[edit]Recently, chemists and those involved in nanotechnology have begun to explore the possibility of creating molecular motors de novo. These synthetic molecular motors currently suffer many limitations that confine their use to the research laboratory. However, many of these limitations may be overcome as our understanding of chemistry and physics at the nanoscale increases. One step toward understanding nanoscale dynamics was made with the study of catalyst diffusion in the Grubb's catalyst system.[16] Other systems like the nanocars, while not technically motors, are also illustrative of recent efforts towards synthetic nanoscale motors.
Other non-reacting molecules can also behave as motors. This has been demonstrated by using dye molecules that move directionally in gradients of polymer solution through favorable hydrophobic interactions.[17] Another recent study has shown that dye molecules, hard and soft colloidal particles are able to move through gradient of polymer solution through excluded volume effects.[18]
See also
[edit]- Brownian motor
- Brownian ratchet
- Cytoskeleton
- Molecular machines
- Molecular mechanics
- Molecular propeller
- Motor proteins
- Nanomotor
- Protein dynamics
- Synthetic molecular motors
References
[edit]- ^ Bustamante C, Chemla YR, Forde NR, Izhaky D (2004). "Mechanical processes in biochemistry". Annual Review of Biochemistry. 73: 705–48. doi:10.1146/annurev.biochem.72.121801.161542. PMID 15189157. S2CID 28061339.
- ^ Nelson P, Radosavljevic M, Bromberg S (2004). Biological physics. Freeman.
- ^ Tsunoda SP, Aggeler R, Yoshida M, Capaldi RA (January 2001). "Rotation of the c subunit oligomer in fully functional F1Fo ATP synthase". Proceedings of the National Academy of Sciences of the United States of America. 98 (3): 898–902. Bibcode:2001PNAS...98..898T. doi:10.1073/pnas.031564198. PMC 14681. PMID 11158567.
- ^ Palma CA, Björk J, Rao F, Kühne D, Klappenberger F, Barth JV (August 2014). "Topological dynamics in supramolecular rotors". Nano Letters. 14 (8): 4461–8. Bibcode:2014NanoL..14.4461P. doi:10.1021/nl5014162. PMID 25078022.
- ^ Dworkin J, Losick R (October 2002). "Does RNA polymerase help drive chromosome segregation in bacteria?". Proceedings of the National Academy of Sciences of the United States of America. 99 (22): 14089–94. Bibcode:2002PNAS...9914089D. doi:10.1073/pnas.182539899. PMC 137841. PMID 12384568.
- ^ Hubscher U, Maga G, Spadari S (2002). "Eukaryotic DNA polymerases". Annual Review of Biochemistry. 71: 133–63. doi:10.1146/annurev.biochem.71.090501.150041. PMID 12045093. S2CID 26171993.
- ^ Peterson CL (November 1994). "The SMC family: novel motor proteins for chromosome condensation?". Cell. 79 (3): 389–92. doi:10.1016/0092-8674(94)90247-X. PMID 7954805. S2CID 28364947.
- ^ Smith DE, Tans SJ, Smith SB, Grimes S, Anderson DL, Bustamante C (October 2001). "The bacteriophage straight phi29 portal motor can package DNA against a large internal force". Nature. 413 (6857): 748–52. Bibcode:2001Natur.413..748S. doi:10.1038/35099581. PMID 11607035. S2CID 4424168.
- ^ Harvey SC (January 2015). "The scrunchworm hypothesis: transitions between A-DNA and B-DNA provide the driving force for genome packaging in double-stranded DNA bacteriophages". Journal of Structural Biology. 189 (1): 1–8. doi:10.1016/j.jsb.2014.11.012. PMC 4357361. PMID 25486612.
- ^ Zhao X, Gentile K, Mohajerani F, Sen A (October 2018). "Powering Motion with Enzymes". Accounts of Chemical Research. 51 (10): 2373–2381. doi:10.1021/acs.accounts.8b00286. PMID 30256612. S2CID 52845451.
- ^ Ghosh S, Somasundar A, Sen A (2021-03-10). "Enzymes as Active Matter". Annual Review of Condensed Matter Physics. 12 (1): 177–200. doi:10.1146/annurev-conmatphys-061020-053036. S2CID 229411011.
- ^ Zhang Y, Hess H (June 2019). "Enhanced Diffusion of Catalytically Active Enzymes". ACS Central Science. 5 (6): 939–948. doi:10.1021/acscentsci.9b00228. PMC 6598160. PMID 31263753.
- ^ Gentile, Kayla; Bhide, Ashlesha; Kauffman, Joshua; Ghosh, Subhadip; Maiti, Subhabrata; Adair, James; Lee, Tae-Hee; Sen, Ayusman (2021-09-22). "Enzyme aggregation and fragmentation induced by catalysis relevant species". Physical Chemistry Chemical Physics. 23 (36): 20709–20717. doi:10.1039/D1CP02966E. ISSN 1463-9084.
- ^ Kay, Euan R.; Leigh, David A.; Zerbetto, Francesco (January 2007). "Synthetic Molecular Motors and Mechanical Machines". Angewandte Chemie International Edition. 46 (1–2): 72–191. doi:10.1002/anie.200504313.
- ^ Lodish H, Berk A, Kaiser CA, Krieger M, Bretscher A, Ploegh H, Amon A, Martin KC (2014). Molecular Cell Biology (8th ed.). New York, NY: w.h.freeman, Macmillan Learning. ISBN 978-1-4641-8339-3.
- ^ Dey KK, Pong FY, Breffke J, Pavlick R, Hatzakis E, Pacheco C, Sen A (January 2016). "Dynamic Coupling at the Ångström Scale". Angewandte Chemie. 55 (3): 1113–7. Bibcode:2016AngCh.128.1125D. doi:10.1002/ange.201509237. PMID 26636667.
- ^ Guha R, Mohajerani F, Collins M, Ghosh S, Sen A, Velegol D (November 2017). "Chemotaxis of Molecular Dyes in Polymer Gradients in Solution". Journal of the American Chemical Society. 139 (44): 15588–15591. doi:10.1021/jacs.7b08783. PMID 29064685.
- ^ Collins M, Mohajerani F, Ghosh S, Guha R, Lee TH, Butler PJ, et al. (August 2019). "Nonuniform Crowding Enhances Transport". ACS Nano. 13 (8): 8946–8956. doi:10.1021/acsnano.9b02811. PMID 31291087. S2CID 195879481.
