Dapansutrile

| |
| Names | |
|---|---|
| Preferred IUPAC name
3-(Methanesulfonyl)propanenitrile | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.255.888 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H7NO2S | |
| Molar mass | 133.17 g·mol−1 |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dapansutrile (OLT1177) is an inhibitor of the NLRP3 (nucleotide-binding domain leucine-rich repeat and pyrin domain containing receptor 3) inflammasome.[1]

An inflammasome can be defined as an immune system receptor that induces inflammation through the activation of caspase 1 and caspase 11 when it is triggered by damaged cells, microbial pathogens, and stress.[3] NLRP3 is a canonical inflammasome.[3] The NLRP3 inflammasome comprises NLRP3, the apoptosis spec-like protein (ASC) and the caspase-1[2] (Figure 1). The NLRP3 inflammasome forms by binding to pattern recognition receptors (PRRs) and damage associated molecular patterns (DAMPS) that activate caspase 1 which then signals for the secretion of pro-inflammatory cytokines IL-1β and IL-18 resulting in pyroptosis[3][4][5] Constant activation of the NLRP3 inflammasome is believed to play a direct or indirect role in acute arthritis, atherosclerosis and various neurodegenerative diseases such as multiple sclerosis (MS), Alzheimer's disease (AD), and Parkinson's disease (PD)[6],[2],.[7] This drug was developed by Olactec Therapeutics with the purpose of decreasing IL-1β peripheral inflammation by binding to the NLRP3 protein and inhibiting the formation of the NLRP3 inflammasome. Interestingly, dapansutrile has also been found to reduce levels of pro inflammatory cytokines IL-18 without interfering with TNF-α levels.[1] Stressed cells in the system can ignite the NLRP3 inflammasome which in turn produces the secretion inflammatory cytokines such as IL-1β and IL-18. Dapansutrile has tested in clinical trials and has been proposed as a beneficial compound for the remedy of osteoarthritis, and gouty arthritis.[1] Nevertheless, other preclinical research has proposed dapansutrile to be potentially beneficial for heart failure and multiple sclerosis.[1]
Molecular Structure and Properties
[edit]Dapansutrile is a β-sulfonyl nitrile compound with four carbon, seven hydrogen, one nitrogen, two oxygen, and one sulfur atom (Figure 2).[8][9] The molecular formula of this compound is C4H7NO2S, and it carries a molecular weight of 133.7 g/mol. Compound acknowledged by the names of dapansutrile, OLT1177, and 3-(methylsulfonyl)propanenitrile.[8]
| Dapansutrile (OLT1177) | |
|---|---|
| Molecular Formula[8] | C4H7NO2S |
| Molecular Weight[10] | 133.7 g/mol |
| IUPAC Name[8] | 3-(methylsulfonyl)propanenitrile |
| Olatec Therapeutics Name[8] | OLT1177 |
| International Non-Proprietary Name[8] | Dapansutrile |
| Storage | Store at -20 °C |
| CAS Number | 54863-37-5 |
| PubChem ID | 12714644 |
| Smiles | C[S](=O)(=O)CCC#N |
| Experimental Solubility[11] | Soluble to:
100 mM in DMSO 20 nM in ethanol |
| Formal Charge[11] | 0 |
| Hydrogen Bond Donor Count[11] | 0 |
| Hydrogen Bond Acceptor Count[11] | 3 |
| Rotatable Bond Count[11] | 2 |
| Topological Polar Surface Area[11] | 66.3 Å2 |
| Heavy Atom Count[11] | 8 |
| Isotope Atom Count[11] | 3 |
Synthesis
[edit]OLT1177 is synthesized by alkylation of sodium methanesulfinate with 3-bromopropionitrile. This reaction produces crude methylsulfonylpropionitrile which is then purified through dissolution into acetone, filtration of the sodium bromide bi-product, solvent exchange via distillation, and recrystallization from ethanol.[9]
Dapansutrile's Mechanism of Action
[edit]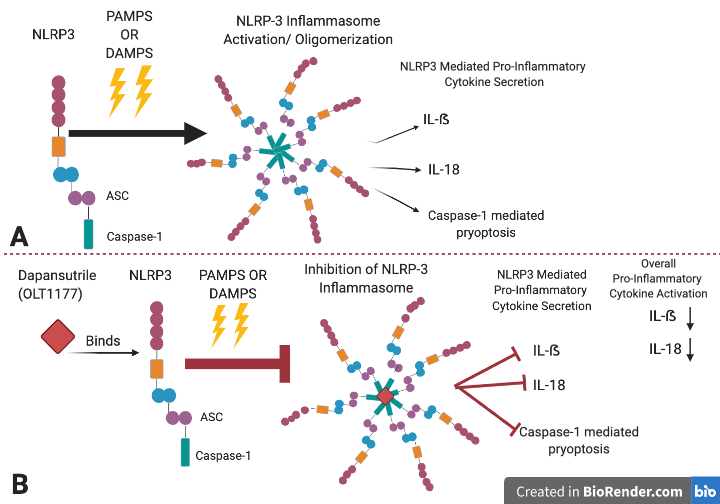
Dapansutrile's Reaction Pathways
[edit]Dapansutrile denoted as a β-sulfonyl nitrile molecule. Its mechanism of action induces a Pinner reaction which is initiated by reacting with thiols, alcohols and amines. Thus, leading to the formation of thiomidates, imidates and amidines respectively.[12] The nitrile group of OLT1177 is still not denoted as a covalent, noncovalent, irreversible or non-reversible inhibitor as there are currently no studies about its reactivity.[9][12] Nevertheless, some researchers believe that dapansutrile promotes inhibition of NLRP3 through covalent bonds (Figure 4).[12]
![Dapansutrile Mechanism of Action. A. Reaction pathway with sulphur. B. Reaction pathway with oxygen. C. Reaction pathway with nitrogen.[12]](http://178.128.105.246/content-http-upload.wikimedia.org/wikipedia/commons/thumb/5/57/Dapansutrile_Mechanism_of_Action.png/1200px-Dapansutrile_Mechanism_of_Action.png) |
|---|
Summary Biological Effects of Dapansutrile
[edit]| Reversible or Non-Reversible | Not Known |
| Primary Mechanism of Action | Inhibits:
|
| Selectivity | Selective to NLRP3 inflammasome |
| IL-1β inhibition % (μM) | 60% (0.0001–10 μM) |
| NLRP3 ATPase inhibition % (μM) | 60% (1 μM) |
| Pyroptosis Inhibition % (μM) | 25–40% (0.001–10 μM) |
| Other Targets | Src; Fyn; HcK; STAT3; NF-κB |
| Cytotoxicity Tc50 ± SE (μM) | Nontoxic in healthy volunteers (up to 1 gram a day for 8 days in oral form) |
| References | [12][9] |
Pharmacokinetics
[edit]Selectivity
[edit]Dapansutrile targets the inhibition of the NLRP3 ATPase and thus blocks the activation of the inflammasome's ASC, and caspase-1 interaction; thereby preventing the assembly of the inflammasome and inflammatory signals such as IL-1β and IL-18. This drug also inhibits pyroptosis. Nevertheless, the drug does not impact the mRNA levels of the NLRP3, ASC, caspase-1, IL-1β, and IL-18 genes.[9]
Research of dapansutrile presents that the compound solely inhibits the NLRP3 inflammasome. Murine macrophages were used and stimulated with lipopolysaccharides and either flagellin or the dsDNA analog Poly(dA:dT) to activate inflammasomes such as NLRC4 and AIM 2 respectively. When Dapansutrile was added they found no difference in the release of TNF-α and IL-1β cytokines to when these inflammasomes were activated.[9]
Other Drug Targets
[edit]Other known targets of dapansutrile include several phosphorylated kinases such as Src; Fyn; HcK; STAT3.[12] Human monocyte derived macrophages (HMDM) cells were stimulated with lipopolysaccharide, and nigericin. Dapansutrile was then added and they found a 26%, 35%, 43% and 33% reduction of these phosphorylated kinases respectively.[9]
Dosages
[edit]In vitro: Human derived macrophages were cultured to study the effect of dapansutrile cytokine production. It was found that dapansutrile at a 1 μM dose inhibited secretion of IL-1β by 60%, and IL-18 levels by 70%. The drug was also found to selectively inhibit pyroptosis at 10 μM.[9]
In vivo: A phase one clinical trial of 35 subjects was conducted to establish the safety of dapansutrile. The daily mean plasma concentration maximum (Cmax) for a single oral dose of dapansutrile was 2,700 ng/mL for the 100-mg dose, 9,800 ng/mL for the 300 mg dose and 32,00 ng/mL for the 1,000 mg dose (Figure 5).[9] Dapansutrile was also studied if given repeatedly once per day for 8 days with a dose of either 100 mg, and 300 mg, or 1,000 mg. The subjects mean plasma concentration on day 8 were 4,800 ng/mL for the 100 mg dose, 15,800 ng/mL for the 300 mg dose and 41,400 ng/mL for the 1,000 mg dose.[9]

| Mean PK Characteristic (units) | 100 mg
N=5 |
300 mg
N=5 |
1000 mg
N=5 |
|---|---|---|---|
| AUC0-t (h*ng/ml) | 76457.07 | 324650.71 | 918963.5 |
| AUC0-24 (h*ng/ml) | 40121.19 | 157378.19 | 461809.56 |
| Residual Area % | 0.71 | 0.74 | 1.12 |
| Cmax (ng/ml) | 2,700 | 9,800 | 32,000 |
| T1/2 el(h) | 23.01 | 22.8 | 24.15 |
| Kel (h) | 0.0309 | 0.0317 | 0.0307 |
| Cl/F (L/h) i.e. oral clearance | 1.32 | 0.97 | 1.11 |
| Vd/F (L) | 43.95 | 31.86 | 37.63 |
| Mean PK Characteristic (units) | N | 100 mg
N=5 |
N | 300 mg | N | 1000 mg |
|---|---|---|---|---|---|---|
| AUC0-τ (h*ng/ml) | 5 | 70288.75 | 3 | 260409.24 | 5 | 7231127.44 |
| Cmax (ng/ml) | 5 | 4,800 | 4 | 15,800 | 5 | 41,400 |
| Cmin(ng/ml) | 5 | 1,710 | 4 | 6,820 | 5 | 18,700 |
| Cave(ng/ml) | 5 | 2,930 | 3 | 10,900 | 5 | 30,100 |
| T1/2 el(h) | 5 | 16.01 | 3 | 21.34 | 5 | 24.72 |
| Clss/F(L/h) | 5 | 1.47 | 3 | 1.17 | 5 | 1.49 |
| Vd/F (L) | 5 | 35.22 | 3 | 36.19 | 5 | 48.56 |
Pharmacodynamics
[edit]Safety and Efficacy of Dapansutrile
[edit]In the Marchetti et al. study, seven of the 35 subjects reported adverse events. Five of the cases were in the single dose study while two cases were in the multi-regimen group. However, the adverse effects were considered unrelated to dapansutrile.[9] Subjects presented no changes in blood pressure (systolic and diastolic), urinalysis, heart rate, liver function enzymes or acute phase proteins in the cohort after an 8-day trial with up to daily 1,000 mg dosing of dapansutrile.[9]
| Single Dose Administration of OLT1177 | Multidose Administration of OLT1177 | ||
|---|---|---|---|
| Dose | Adverse Event | Dose | Adverse Event |
| Placebo | Headache | 100 mg | Diarrhea |
| 100 mg | Eczema | 100 mg | Headache |
| 1000 mg | Migraine |
|
|
| 1000 mg | Back pain |
|
|
Potential Therapeutic Applications
[edit]Neurodegenerative diseases
[edit]Multiple Sclerosis:
Multiple sclerosis (MS) is a neurodegenerative disease characterized by the immune system deteriorating myelin. Myelin damage leads to the disruption of neuronal signaling, and dysregulated inflammatory levels.[13] Dapansutrile was used in the Experimental Autoimmune Encephalomyelitis (EAE) mouse model to understand its possible underlying effects for MS. It was found that mice fed with the dapansutrile diet protected the mice from demyelination in the spinal cord as well as decreased their levels of interleukins IL-1β and IL-18.[14] Currently, it is unclear whether the drug could inhibit microglial reactivity, but currently it has no known benefits to aid in the prevention of dementia and cognitive function.[1]
Inflammation
[edit]Gouty Arthritis:
Gouty arthritis is an inflammatory joint disorder, partly induced to the activation of the NLRP3 inflammasome and excess IL-1β activation which leads to gout attacks.[15] This is due to excess uric acid in the blood which promotes the formation of uric acid crystals in the joints. Uric acid is a known danger signal and induce the cleavage signal of the caspase-1 NLRP3 inflammasome.[16]
Animal Model Experiments: In 2018, two murine models were used to validate Dapansutrile as a drug beneficial for acute joint inflammation. They injected zymosan or monosodium urate crystals in order to induce gouty arthritis in mice. OLT1177 was then either injected intraperitoneally or through their diet. In essence, the drug reduced joint inflammation, as well as interleukin levels[6] demonstrating its therapeutic benefit for this disease.
Clinical Trials: As for clinical trials, Olatec Therapeutics also conducted a phase 2.a trials where Dapansutrile was given to 29 subjects with gouty arthritis.[1]
Cardiovascular Diseases
[edit]Acute Myocardial Infarction/ Heart Attack:
One of the major downstream effects of having coronary artery disease is possibility of having an acute myocardial infarction or heart attack.[2] In mouse models, Dapansutrile was found to decrease infarct size a dose-dependent manner.[2]
Heart Failure:
Olatec Therapeutics conducted a phase 1 clinical trial for Dapansutrile as a potential therapeutic for systolic heart failure.[17] They have carried out a Phase 1 double blinded study with a total of 30 subjects to assess the drug's safety and pharmacodynamics. The drug was given in capsule form and the subjects were divided into 3 cohorts. Each cohort had 8 subjects taking an oral capsule with 100 mg Dapansutrile while 2 subjects were given the placebo capsule. Although the completion date of this clinical trial was on November 21, 2019, the results of the study have not yet been published.[17]
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) / COVID-19:
Dapansutrile has been proposed by a number of scientists as a measure to reduce cardiovascular outcomes that seem to be brought on by COVID-19.[18] The rationale behind using Dapansutrile is to inhibit the NLRP3 inflammasome and reduce the chances of a cytokine storm which seems to cause multi-organ failure in COVID-19 patients.[18] Subjects with COVID-19 have shown to have an increased concentration of pro-inflammatory cytokines resulting in the cytokine storm, and thus producing an exhaustion of lymphocytes.[19] NLRP3 not only activates cytokines but other key players that can inflict myocardial damages, and Acute Respiratory Distress Syndrome (ARDS), and its inhibition has been found to deter these outcomes.[18][20] Thus, dapansutrile is proposed as a possible mediator to relief and prevent COVID-19 symptoms and effects.
References
[edit]- ^ a b c d e f "Dapansutrile" (PDF). Alzheimer's Drug Discovery Foundation. Archived (PDF) from the original on 2021-08-22.
- ^ a b c d e f Silvis, Max J. M.; Demkes, Evelyne J.; Fiolet, Aernoud T. L.; Dekker, Mirthe; Bosch, Lena; van Hout, Gerardus P. J.; Timmers, Leo; de Kleijn, Dominique P. V. (2020-07-09). "Immunomodulation of the NLRP3 Inflammasome in Atherosclerosis, Coronary Artery Disease, and Acute Myocardial Infarction". Journal of Cardiovascular Translational Research. 14 (1): 23–34. doi:10.1007/s12265-020-10049-w. ISSN 1937-5395. PMC 7892681. PMID 32648087.
- ^ a b c Lamkanfi, Mohamed; Dixit, Vishva M. (2014-05-22). "Mechanisms and Functions of Inflammasomes". Cell. 157 (5): 1013–1022. doi:10.1016/j.cell.2014.04.007. ISSN 0092-8674. PMID 24855941.
- ^ Swanson, Karen V.; Deng, Meng; Ting, Jenny P.-Y. (August 2019). "The NLRP3 inflammasome: molecular activation and regulation to therapeutics". Nature Reviews Immunology. 19 (8): 477–489. doi:10.1038/s41577-019-0165-0. ISSN 1474-1741. PMC 7807242. PMID 31036962.
- ^ a b Zheng, Danping; Liwinski, Timur; Elinav, Eran (2020-06-09). "Inflammasome activation and regulation: toward a better understanding of complex mechanisms". Cell Discovery. 6 (1): 36. doi:10.1038/s41421-020-0167-x. ISSN 2056-5968. PMC 7280307. PMID 32550001.
- ^ a b Marchetti, Carlo; Swartzwelter, Benjamin; Koenders, Marije I.; Azam, Tania; Tengesdal, Isak W.; Powers, Nick; de Graaf, Dennis M.; Dinarello, Charles A.; Joosten, Leo A. B. (December 2018). "NLRP3 inflammasome inhibitor OLT1177 suppresses joint inflammation in murine models of acute arthritis". Arthritis Research & Therapy. 20 (1): 169. doi:10.1186/s13075-018-1664-2. ISSN 1478-6362. PMC 6091035. PMID 30075804.
- ^ Guan, Yadi; Han, Fang (2020). "Key Mechanisms and Potential Targets of the NLRP3 Inflammasome in Neurodegenerative Diseases". Frontiers in Integrative Neuroscience. 14: 37. doi:10.3389/fnint.2020.00037. ISSN 1662-5145. PMC 7393579. PMID 32792920.
- ^ a b c d e f "dapansutrile | C4H7NO2S | ChemSpider". www.chemspider.com. Retrieved 2020-11-15.
- ^ a b c d e f g h i j k l m n o p q Marchetti, Carlo; Swartzwelter, Benjamin; Gamboni, Fabia; Neff, Charles P.; Richter, Katrin; Azam, Tania; Carta, Sonia; Tengesdal, Isak; Nemkov, Travis; D'Alessandro, Angelo; Henry, Curtis (13 February 2018). "OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation". Proceedings of the National Academy of Sciences of the United States of America. 115 (7): E1530–E1539. Bibcode:2018PNAS..115E1530M. doi:10.1073/pnas.1716095115. ISSN 1091-6490. PMC 5816172. PMID 29378952.
- ^ a b PubChem. "Dapansutrile". pubchem.ncbi.nlm.nih.gov. Retrieved 2020-11-29.
- ^ a b c d e f g h PubChem. "Dapansutrile". pubchem.ncbi.nlm.nih.gov. Retrieved 2020-10-19.
- ^ a b c d e f g Bertinaria, Massimo; Gastaldi, Simone; Marini, Elisabetta; Giorgis, Marta (2019-07-30). "Development of covalent NLRP3 inflammasome inhibitors: Chemistry and biological activity". Archives of Biochemistry and Biophysics. Inflammasomes: Intracellular mediators of immune defence. 670: 116–139. doi:10.1016/j.abb.2018.11.013. hdl:2318/1720025. ISSN 0003-9861. PMID 30448387. S2CID 53760314.
- ^ Dobson, R.; Giovannoni, G. (2019). "Multiple sclerosis – a review". European Journal of Neurology. 26 (1): 27–40. doi:10.1111/ene.13819. ISSN 1468-1331. PMC 1589931. PMID 30300457.
- ^ Sánchez-Fernández, Alba; Skouras, Damaris B.; Dinarello, Charles A.; López-Vales, Rubèn (2019). "OLT1177 (Dapansutrile), a Selective NLRP3 Inflammasome Inhibitor, Ameliorates Experimental Autoimmune Encephalomyelitis Pathogenesis". Frontiers in Immunology. 10: 2578. doi:10.3389/fimmu.2019.02578. ISSN 1664-3224. PMC 6839275. PMID 31736980.
- ^ Ragab, Gaafar; Elshahaly, Mohsen; Bardin, Thomas (2017-09-01). "Gout: An old disease in new perspective – A review". Journal of Advanced Research. 8 (5): 495–511. doi:10.1016/j.jare.2017.04.008. ISSN 2090-1232. PMC 5512152. PMID 28748116.
- ^ Wen, Haitao; Ting, Jenny P.-Y.; O'Neill, Luke A. J. (April 2012). "A role for the NLRP3 inflammasome in metabolic diseases—did Warburg miss inflammation?". Nature Immunology. 13 (4): 352–357. doi:10.1038/ni.2228. ISSN 1529-2916. PMC 4090390. PMID 22430788.
- ^ a b Olatec Therapeutics LLC (2020-01-06). "A Phase 1b, Randomized, Double-Blinded, Dose Escalation, Single Center, Repeat-Dose Safety and Pharmacodynamics Study of Orally Administered Dapansutrile Capsules in Subjects With NYHA II-III Systolic Heart Failure".
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b c "SARS-CoV-2 infection: NLRP3 inflammasome as plausible target to prevent cardiopulmonary complications?". European Review. 2020-09-14. Retrieved 2020-11-15.
- ^ Tang, Yujun; Liu, Jiajia; Zhang, Dingyi; Xu, Zhenghao; Ji, Jinjun; Wen, Chengping (2020-07-10). "Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies". Frontiers in Immunology. 11: 1708. doi:10.3389/fimmu.2020.01708. ISSN 1664-3224. PMC 7365923. PMID 32754163.
- ^ Zahid, Ayesha; Li, Bofeng; Kombe, Arnaud John Kombe; Jin, Tengchuan; Tao, Jinhui (2019). "Pharmacological Inhibitors of the NLRP3 Inflammasome". Frontiers in Immunology. 10: 2538. doi:10.3389/fimmu.2019.02538. ISSN 1664-3224. PMC 6842943. PMID 31749805.

![Figure 1. Molecular structure of dapansutrile[10]](http://178.128.105.246/content-http-upload.wikimedia.org/wikipedia/commons/1/11/Chemical_Structure_of_OTL1177.png)