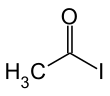
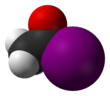
Acetyl iodide
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Acetyl iodide[1] | |||
| Systematic IUPAC name
Ethanoyl iodide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.007.330 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| UN number | 1898 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H3IO | |||
| Molar mass | 169.949 g·mol−1 | ||
| Boiling point | 108 °C; 226 °F; 381 K | ||
| Decomposes | |||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
-163.18--161.42 kJ mol−1 | ||
| Related compounds | |||
Related acyl halides
|
Acetyl chloride | ||
Related compounds
|
Acetic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Acetyl iodide is an organoiodine compound with the formula CH3COI. It is a colourless liquid. It is formally derived from acetic acid. Although far rarer in the laboratory than the related acetyl bromide and acetyl chloride, acetyl iodide is produced, transiently at least, on a far larger scale than any other acid halide. Specifically, it is generated by the carbonylation of methyl iodide in the Cativa and Monsanto processes, which are the main industrial processes that generate acetic acid.[2] It is also an intermediate in the production of acetic anhydride from methyl acetate.[3]
Upon treatment with carboxylic acids, acetyl iodide does not exhibit reactions typical of acyl halides, such as acetyl chloride. Instead, acetyl iodide undergoes iodide/hydroxide exchange with most carboxylic acids:[4]
- CH3COI + RCO2H → CH3CO2H + RCOI
References
[edit]- ^ "ACETYL IODIDE - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ^ Jones, J. H. (2000). "The Cativa Process for the Manufacture of Acetic Acid" (PDF). Platinum Metals Rev. 44 (3): 94–105. Archived from the original (PDF) on 2015-09-24. Retrieved 2011-07-03.
- ^ Zoeller, J. R.; Agreda, V. H.; Cook, S. L.; Lafferty, N. L.; Polichnowski, S. W.; Pond, D. M. (1992), "Eastman Chemical Company Acetic Anhydride Process", Catal. Today, 13 (1): 73–91, doi:10.1016/0920-5861(92)80188-S
- ^ M. G. Voronkov; L. I. Belousova; A. A. Trukhina; N. N. Vlasova (2003). "Acyl Iodides in Organic Synthesis: IV. Reaction of Acetyl Iodide with Carboxylic Acids". Russian Journal of Organic Chemistry. 39 (12): 1702. doi:10.1023/B:RUJO.0000019730.43667.46. S2CID 97920158.