Carbenium ion
It has been suggested that Carbocation be merged into this article. (Discuss) Proposed since August 2024. |

A carbenium ion is a positive ion with the structure RR′R″C+, that is, a chemical species with carbon atom having three covalent bonds, and it bears a +1 formal charge. Carbenium ions are a major subset of carbocations, which is a general term for diamagnetic carbon-based cations. In parallel with carbenium ions is another subset of carbocations, the carbonium ions with the formula R5+. Methenium, CH+
3 is the parent member.
Carbenium ions are generally highly reactive due to having an incomplete octet of electrons; however, certain carbenium ions, such as the tropylium ion, are relatively stable due to the positive charge being delocalised between the carbon atoms.
Nomenclature
[edit]Reactivity
[edit]Stability
[edit]Since carbenium ions can be highly reactive, a major consideration is their stability. The stability of carbenium ions correlates with the electron-donating properties of the substituents. Trialkylcarbenium ions, such as (CH3)3C+, are isolable as salts, but H3C+ cannot. An analogous situation applies to triarylcarbenium ions: salts of triphenylcarbenium (C5H5)3C+ are readily isolable (see trityl), and those with amine substituents so robust that they are used as dyes, e.g. crystal violet. Carbenium ions can also be stabilized by conjugation to double bonds giving allyl cations, which enjoy some resonance stabilization. This situation is illustrated by the isolation of protonated benzene.[2] Lone-pair bearing heteroatoms also stabilize carbenium ions.[3]
Rearrangements
[edit]Carbenium ions sometimes rearranges readily. For example, when pentan-3-ol is heated with aqueous HCl, the initially formed 3-pentyl carbocation rearranges to a mixture of the 3-pentyl and 2-pentyl. These cations react with chloride ion to produce 3-chloropentane and 2-chloropentane in a ratio of approximately 1:2. [4]
Types of carbenium ions
[edit]Alkylium ions
[edit]Carbenium ions can be prepared directly from alkanes by removing a hydride anion, H−
, with a strong acid. For example, magic acid, a mixture of antimony pentafluoride (SbF
5) and fluorosulfuric acid (FSO
3H), turns isobutane into the trimethylcarbenium cation, (CH
3)
3C+
.[5][6]
Aromatic carbenium ions
[edit]
The tropylium ion is an aromatic species with the formula C
7H+
7.[7] Its name derives from the molecule tropine (itself named for the molecule atropine). Salts of the tropylium cation can be stable, e.g. tropylium tetrafluoroborate. It can be made from cycloheptatriene (tropylidene) and bromine or phosphorus pentachloride.[8]
It is a planar, cyclic, heptagonal ion; it also has 6 π-electrons (4n + 2, where n = 1), which fulfills Hückel's rule of aromaticity. It can coordinate as a ligand to metal atoms.
The structure shown is a composite of seven resonance contributors in which each carbon carries part of the positive charge.
In 1891 G. Merling obtained a water-soluble salt from a reaction of cycloheptatriene and bromine.[9] The structure was elucidated by Eggers Doering and Knox in 1954.[10][11]
Another aromatic carbenium ion is the cyclopropenyl or cyclopropenium ion, C
3H+
3, obtained by Ronald Breslow and John T. Groves in 1970.[12] Though less stable than the tropylium cation, this carbenium ion can also form salts at room temperature. Solutions of such salts were found by Breslow and Groves to have spectroscopic and chemical properties matching expectations for an aromatic carbenium ion.
Arenium ions
[edit]An arenium ion is a cyclohexadienyl cation that appears as a reactive intermediate in electrophilic aromatic substitution.[13] For historic reasons this complex is also called a Wheland intermediate,[14] or a σ-complex.
Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring.[15] The arenium ion is no longer an aromatic species; however it is relatively stable due to delocalization: the positive charge is delocalized over 5 carbon atoms. Also contributing to the stability of arenium ions is the energy gain resulting from the strong C-e bond (E = electrophile).
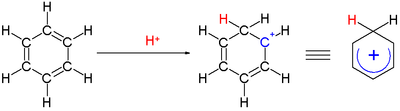
The smallest arenium ion is protonated benzene, C
6H+
7. The benzenium ion can be isolated as a stable compound when benzene is protonated by the carborane superacid, H(CB11H(CH3)5Br6).[16] The benzenium salt is crystalline with thermal stability up to 150 °C. Bond lengths deduced from X-ray crystallography are consistent with a cyclohexadienyl cation structure.
Cyclopropylmethyl cation
[edit]A non-classical structure for C
4H+
7 is supported by substantial experimental evidence from solvolysis experiments and NMR studies. One or both of two structures, the cyclopropylcarbinyl cation and the bicyclobutonium cation, were invoked to account for the observed reactivity in various experiments, while the NMR data point to a highly fluxional system that undergoes rapid rearrangement to give an averaged spectrum consisting of only two 13C NMR signals, even at temperatures as low as −132 °C. Computationally, it was confirmed that the energetic landscape of the C
4H+
7 system is very flat, and that the two isomers postulated based on experimental data are very close in energy, the bicyclobutonium structure being computed to be just 0.4 kcal/mol more stable than the cyclopropylcarbinyl structure. In the solution phase (SbF5·SO2ClF·SO2F2, with SbF–
6 as the counterion), the bicyclobutonium structure predominates over the cyclopropylcarbinyl structure in a 84:16 ratio at −61 °C.
Three other possible structures, two classical structures (the homoallyl cation and cyclobutyl cation) and a more highly delocalized non-classical structure (the tricyclobutonium ion), are now known to be less stable isomers (or merely a transition state rather than an energy minimum in the case of the cyclobutyl cation).[17]

Substituted cyclopropylcarbinyl cations have also been studied by NMR:[18][19]
In the NMR spectrum of a dimethyl derivative, two nonequivalent signals are found for the two methyl groups, indicating that the molecular conformation of this cation is not perpendicular (as in A), which possesses a mirror plane, but is bisected (as in B) with the empty p-orbital parallel to the cyclopropyl ring system:
In terms of bent bond theory, this preference is explained by assuming favorable orbital overlap between the filled cyclopropane bent bonds and the empty p-orbital.[20]
Acylium ions
[edit]An acylium ion is a cation with the formula RCO+.[21] The structure is described as R−C≡O+ or R−=O. It is an acyl carbocation, but the actual structure has the oxygen and carbon linked by a triple bond. Such species are common reactive intermediates, for example, in the Friedel−Crafts acylations also in many other organic reactions such as the Hayashi rearrangement. Salts containing acylium ions can be generated by removal of the halide from acyl halides:
- RCOCl + SbCl5 → RCO+SbCl−
6
The C–O distance in these cations is near 1.1 ångströms, even shorter than that in carbon monoxide.[22] Acylium cations are characteristic fragments observed in EI-mass spectra of ketones.
Selected applications
[edit]
Carbenium ions are so integrated into organic chemistry that a full inventory of their commercially useful reactions would be long.
The alkylation of benzene with alpha-olefins to give linear alkylbenzene (LABs) illustrates the behaviour of secondary carbenium ions. The alkylation is initiated by strong acids. LABs are a key precursor to detergents.
Derivatives of the triphenylcarbenium are the triarylmethane dyes.[23]
See also
[edit]References
[edit]- ^ Scholz, Franziska; Himmel, Daniel; Scherer, Harald; Krossing, Ingo (2013). "Superacidic or Not…︁? Synthesis, Characterisation, and Acidity of the Room-Temperature Ionic Liquid [C(CH3)3]+ [Al2Br7]−". Chemistry – A European Journal. 19 (1): 109–116. doi:10.1002/chem.201203260. PMID 23180742.
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 239, ISBN 978-0-471-72091-1
- ^ Hansjörg Grützmacher, Christina M. Marchand (1997), "Heteroatom stabilized carbenium ions", Coord. Chem. Rev., 163, 287–344. doi:10.1016/S0010-8545(97)00043-X
- ^ March
- ^ George A. Olah and Joachim Lukas (1967), "Stable Carbonium Ions. XLVII. Alkylcarbonium ion formation from alkanes via hydride (alkide) ion abstraction in fluorosulfonic acid-antimony pentafluoride-sulfuryl chlorofluoride solution". J. Am. Chem. Soc. 89 (18), 4739–4744 doi:10.1021/ja00994a030
- ^ Kato, Tsuyoshi; Reed, Christopher A. (2004). "Putting tert -Butyl Cation in a Bottle". Angewandte Chemie International Edition. 43 (22): 2908–2911. doi:10.1002/anie.200453931. PMID 15170300.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "molecule". doi:10.1351/goldbook.M04002
- ^ "Tropylium tetrafluorate" Organic Syntheses, Coll. Vol. 5, p.1138 (1973); Vol. 43, p.101 (1963). link Archived 2012-08-29 at the Wayback Machine
- ^ Merling, G. (1891), "Ueber Tropin". Berichte der deutschen chemischen Gesellschaft, 24: 3108–3126. doi:10.1002/cber.189102402151
- ^ "The Cycloheptatrienylium (Tropylium) Ion" W. von E. Doering, L. H. Knox J. Am. Chem. Soc., 1954, 76 (12), pp 3203–3206 doi:10.1021/ja01641a027
- ^ "Aromaticity as a Cornerstone of Heterocyclic Chemistry" Alexandru T. Balaban, Daniela C. Oniciu, Alan R. Katritzky Chem. Rev., 2004, 104 (5), 2777–2812 doi:10.1021/cr0306790
- ^ "Cyclopropenyl Cation. Synthesis and Characterization." R. Breslow and J. T. Groves J. Am. Chem. Soc., 1970, 92 (4), 984–987 [1]
- ^ Olah, George A. (1972). "Stable carbocations. CXVIII. General concept and structure of carbocations based on differentiation of trivalent (classical) carbenium ions from three-center bound penta- of tetracoordinated (nonclassical) carbonium ions. Role of carbocations in electrophilic reactions". Journal of the American Chemical Society. 94 (3): 808–820. doi:10.1021/ja00758a020. ISSN 0002-7863.
- ^ "A Quantum Mechanical Investigation of the Orientation of Substituents in Aromatic Molecules" G. W. Wheland J. Am. Chem. Soc.; 1942; 64(4) 900–908; doi:10.1021/ja01256a047
- ^ A guidebook to mechanism in organic chemistry, Peter Sykes; pp 130–133
- ^ "Isolating Benzenium Ion Salts" Christopher A. Reed, Kee-Chan Kim, Evgenii S. Stoyanov, Daniel Stasko, Fook S. Tham, Leonard J. Mueller, and Peter D. W. Boyd J. Am. Chem. Soc.; 2003; 125(7) 1796–1804; doi:10.1021/ja027336o
- ^ Olah, George A.; Surya Prakash, G. K.; Rasul, Golam (July 2008). "Ab Initio/GIAO-CCSD(T) Study of Structures, Energies, and 13C NMR Chemical Shifts of C
4H+
7 and C
5H+
9 Ions: Relative Stability and Dynamic Aspects of the Cyclopropylcarbinyl vs Bicyclobutonium Ions". Journal of the American Chemical Society. 130 (28): 9168–9172. doi:10.1021/ja802445s. ISSN 0002-7863. PMID 18570420. - ^ Kabakoff, David S.; Namanworth, Eli (1970). "Nuclear magnetic double resonance studies of the dimethylcyclopropylcarbinyl cation. Measurement of the rotation barrier". Journal of the American Chemical Society. 92 (10): 3234–3235. doi:10.1021/ja00713a080.
- ^ Pittman Jr., Charles U.; Olah, George A. (1965). "Stable Carbonium Ions. XVII.1a Cyclopropyl Carbonium Ions and Protonated Cyclopropyl Ketones". Journal of the American Chemical Society. 87 (22): 5123–5132. doi:10.1021/ja00950a026.
- ^ Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry Part A (2nd ed.).
- ^ Compendium of Chemical Terminology, acyl groups
- ^ Chevrier, B.; Le Carpentier, J. M.; Weiss, R. (1972). "Synthesis of two crystalline species of the Friedel–Crafts intermediate antimony pentachloride-p-toluoyl chloride. Crystal structures of the donor–acceptor complex and of the ionic salt". J. Am. Chem. Soc. 94 (16): 5718–5723. doi:10.1021/ja00771a031.
- ^ Gessner, Thomas; Mayer, Udo (2000). "Triarylmethane and Diarylmethane Dyes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_179. ISBN 978-3527306732.