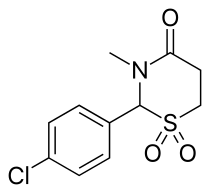
Chlormezanone
Appearance
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 40.5 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.190 |
| Chemical and physical data | |
| Formula | C11H12ClNO3S |
| Molar mass | 273.73 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Chlormezanone (marketed under the brandname Trancopal or Fenaprim) is a drug used as an anxiolytic and a muscle relaxant.[1]
Its use was discontinued in many countries in 1996 due to rare but serious cases of toxic epidermal necrolysis.[2]
Synthesis
[edit]
References
[edit]- ^ Turner P, Volan G, Wiseman H (1997). Drugs Handbook. UK: Palgrave Macmillan. p. 20. ISBN 978-1-349-13937-8.
- ^ Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, Harr T (February 2018). "Current Perspectives on Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis". Clinical Reviews in Allergy & Immunology. 54 (1): 147–176. doi:10.1007/s12016-017-8654-z. PMID 29188475. S2CID 46796285.
- ^ US 3082209, Surrey AR, "4-metathiazanone derivatives and their preparation", issued 1958, assigned to Sterling Drug Inc.
- ^ Surrey AR, Webb WG, Gesler RM (1958). "Central Nervous System Depressants. The Preparation of Some 2-Aryl-4-metathiazanones". Journal of the American Chemical Society. 80 (13): 3469–3471. doi:10.1021/ja01546a065.
Further reading
[edit]- Wollina U, Hipler UC, Seeling A, Oelschlager H (2005). "Investigations of interactions of chlormezanone racemate and its enantiomers on human keratinocytes and human leucoytes in vitro". Skin Pharmacology and Physiology. 18 (3): 132–138. doi:10.1159/000084910. PMID 15897685. S2CID 36642315.
- Seeling A, Oelschläger H, Rothley D (April 2000). "[Important pharmaceutical-chemical characteristics of the central muscle relaxant chlormezanone]". Die Pharmazie. 55 (4): 293–296. PMID 10798243.
- Oelschläger H, Klinger W, Rothley D, Seeling A, Bockhard H, Hofmann B, et al. (September 1998). "[Cleavage and biotransformation of the central muscle relaxant chlormezanone]". Die Pharmazie. 53 (9): 620–624. PMID 9770210.
- Gautier V, Vinçon G, Demotes-Mainard F, Albin H (1990). "[Pharmacokinetics of chlormezanone in healthy volunteers]". Therapie. 45 (4): 315–319. PMID 2399514.
