Lugdunin

| |
| Names | |
|---|---|
| IUPAC name
(1R,4R,7S,10R,13S,16R,19S)-7-(1H-Indol-3-ylmethyl)-10-isobutyl-4,13,16,19-tetraisopropyl-21-thia-3,6,9,12,15,18,23-heptaazabicyclo[18.2.1]tricosane-2,5,8,11,14,17-hexone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C40H62N8O6S | |
| Molar mass | 783.05 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lugdunin is an investigational antibiotic, classified as a thiazolidine-containing cyclic peptide. It was isolated in 2016 after Staphylococcus lugdunensis was identified as the species of bacteria from the human nose that suppressed growth of species of disease-causing bacteria in that part of the human microbiome.[1][2][3]
Lugdunin is a non-ribosomally synthesized cyclic peptide that inhibits growth of Staphylococcus aureus strains. The lugdunin genes are located on a 30-kbp operon. The genes lugA, lugB, lugC, and lugD encode four non-ribosomal peptide synthases, which are preceded by a putative regulator gene lugR.[4]
| Gene | locustag | protein size/aa | Genbank protein entry | RefSeq protein entry |
|---|---|---|---|---|
| lugR | SLUG_RS03935 | 196 | CCB53263.1 | WP_002460032.1 |
| lugA | SLUG_RS03940 | 2374 | CCB53264.1 | WP_002478842.1 |
| SLUG_RS03945 | 124 | CCB53265.1 | WP_002460029.1 | |
| lugB | SLUG_RS03950 | 1230 | CCB53266.1 | WP_014533237.1 |
| lugC | SLUG_RS03955 | 2937 | CCB53267.1 | WP_002478844.1 |
| lugT | SLUG_RS03960 | 228 | CCB53268.1 | WP_002460022.1 |
| lugD | SLUG_RS03965 | 579 | CCB53269.1 | WP_002478846.1 |
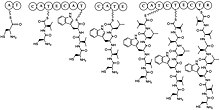
Biosynthesis
[edit]Lugdunin is synthesized by non ribosomal peptide synthetases in S. lugdunensis. The molecule is a cyclic peptide composed of a thiazolidine heterocycle and three D amino acids. The operon responsible for lugdunin synthesis is approximately 30 kb and contains four non ribosomal peptide synthetase genes. The operon contains a phosphopantetheinyl transferase, monooxygenase, an unknown tailoring enzyme, a regulator gene, and a type II thioesterase.[5] Phosphopantetheinyl transferases carry out the activation of T domains, which act as carrier proteins. Monooxygenases incorporate a single hydroxyl into a lugdunin intermediate. The type II thioesterase is utilized to remove intermediates that stall during biosynthesis.[citation needed]
A surprising note about lugdunin is that the operon only encodes five adenylation domains, an interestingly small amount for such a large molecule. This discrepancy is accounted for by the addition of three consecutive valine residues in alternating D and L configurations by LugC. The thiazolidine ring forms following the release of the metabolite via reduction. The N-terminal L-Cysteine residue nucleophilically attacks the carbonyl[6] on the C-terminal L-valine residue, thus forming an imine macrocycle. The Schiff base formed in this reaction is then nucleophilically attacked by a cysteine thiol which produces the thiazolidine heterocycle previously described.[citation needed]
References
[edit]- ^ Gallagher, James (2016-07-27). "Antibiotic resistance: 'Snot wars' study heralds new class of drugs". BBC News. Retrieved 2016-07-27.
- ^ Zipperer, Alexander; Konnerth, Martin C.; Laux, Claudia; Berscheid, Anne; Janek, Daniela; Weidenmaier, Christopher; Burian, Marc; Schilling, Nadine A.; Slavetinsky, Christoph (2016). "Human commensals producing a novel antibiotic impair pathogen colonization". Nature. 535 (7613): 511–516. Bibcode:2016Natur.535..511Z. doi:10.1038/nature18634. PMID 27466123. S2CID 205249755.
- ^ "Scientists find microbiotic treasure hidden in the nose". Los Angeles Times. 2016-07-27. Retrieved 2016-07-27.
- ^ Krismer, Bernhard; Peschel, Andreas; Grond, Stephanie; Brötz-Oesterhelt, Heike; Schittek, Birgit; Kalbacher, Hubert; Willmann, Matthias; Marschal, Matthias; Slavetinsky, Christoph; Schilling, Nadine A.; Burian, Marc; Weidenmaier, Christopher; Janek, Daniela; Berscheid, Anne; Laux, Claudia; Konnerth, Martin C.; Zipperer, Alexander (July 2016). "Extended Data Figure 1: Gene cluster of lugdunin and generation of S. lugdunensis IVK28-Xyl". Nature. 535 (7613): 511–516. Bibcode:2016Natur.535..511Z. doi:10.1038/nature18634. PMID 27466123. S2CID 205249755.
- ^ Krauss, Sophia; Zipperer, Alexander; Wirtz, Sebastian; Saur, Julian; Konnerth, Martin C.; Heilbronner, Simon; Torres Salazar, Benjamin O.; Grond, Stephanie; Krismer, Bernhard; Peschel, Andreas (2020-12-16). "Secretion of and Self-Resistance to the Novel Fibupeptide Antimicrobial Lugdunin by Distinct ABC Transporters in Staphylococcus lugdunensis". Antimicrobial Agents and Chemotherapy. 65 (1): e01734–20. doi:10.1128/AAC.01734-20. ISSN 0066-4804. PMC 7927808. PMID 33106269.
- ^ "Lugdunin - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2022-06-04.
External links
[edit]- "Press release: A potential lifesaver lies unrecognized in the human body" (PDF). www.uni-tuebingen.de. University of Tübingen. Retrieved 2016-07-28.
