PDK4
Pyruvate dehydrogenase lipoamide kinase isozyme 4, mitochondrial (PDK4) is an enzyme that in humans is encoded by the PDK4 gene.[5][6] It codes for an isozyme of pyruvate dehydrogenase kinase.
This gene is a member of the PDK/BCKDK protein kinase family and encodes a mitochondrial protein with a histidine kinase domain. This protein is located in the matrix of the mitochondria and inhibits the pyruvate dehydrogenase complex by phosphorylating one of its subunits, reducing the conversion of pyruvate, which is produced from the oxidation of glucose and amino acids, to acetyl-CoA and contributing to the regulation of glucose metabolism. Expression of this gene is regulated by glucocorticoids, retinoic acid and insulin.[6] PDK4 is increased in hibernation and helps to decrease metabolism and conserve glucose by decreasing its conversion to acetyl-CoA, which enters the citric acid cycle and is converted to ATP.[7]
Structure
[edit]The mature protein encoded by the PDK4 gene contains 294 amino acids in its sequence. To form the active protein, two of the polypeptide chains come together to form an open conformation.[6] Specifically, the two subunits come together to form a nucleotide-binding pocket; this pocket is targeted most often by inhibitors.[8]
Function
[edit]The pyruvate dehydrogenase (PDH) complex must be tightly regulated due to its central role in general metabolism. Within the complex, there are three serine residues on the E1 component that are sites for phosphorylation; this phosphorylation inactivates the complex. In humans, there have been four isozymes of pyruvate dehydrogenase kinase that have been shown to phosphorylate these three sites: PDK1, PDK2, PDK3, and PDK4. PDK4 does not incorporate the most phosphate groups per catalytic event, because it can only phosphorylate site 1 and site 2; its rate of phosphorylation is less than PDK1, equal to PDK3, and more than PDK2. When the thiamine pyrophosphate (TPP) coenzyme is bound, the rates of phosphorylation by all four isozymes are drastically affected. Site 1 is the most affected, with the rate being significantly decreased. However, overall activity by PDK4 is not affected.[9]
Regulation
[edit]As the primary regulators of a crucial step in the central metabolic pathway, the pyruvate dehydrogenase family is tightly regulated itself by a myriad of factors including transcription factors Sp1 and CCAAT box binding factor (CBF). Retinoic acid enhances PDK4 transcription by enabling retinoic acid receptor family members to recruit transcriptional coactivators to retinoic acid response elements (RAREs) in the PDK4 promoter. Transcription is also increased by inhibiting inhibitory histone deacetylases (HDACs) using trichostatin A (TSA).[10] Rosiglitazone, a thiazolidinedione known to activate the glycerol biogenesis pathway, increases PDK4 mRNA transcription in white adipose tissue, but not in liver or muscle tissue.[11] Farnesoid X receptor, or FXR, suppresses glycolysis and enhances fatty acid oxidation by increasing PDK4 expression and inactivating the PDH complex.[12] Other factors, such as insulin, directly downregulate both PDK2 and PDK4 mRNA transcription. This is done through a proposed phosphatidylinositol 3-kinase (PI3K)-dependent pathway. In fact, even when cells are exposed to dexamethasone to increase mRNA expression, insulin blocks this effect.[13] Peroxisome proliferator-activated receptors also regulate expression; PPAR alpha and delta were found to upregulate PDK4 mRNA, but PPAR gamma activation downregulated expression.[14]
Clinical significance
[edit]PDK4 is relevant in a variety of clinical conditions. Short-term fasting induces an increase in PDK4 transcription by about 10-fold.[15] Upon refeeding, transcription of PDK4 increased further, a surprising outlook, by about 50-fold over levels before fasting began.[16] This effect can be seen long term as well. PDK4 is overexpressed in skeletal muscle in type 2 diabetes, resulting in impaired glucose utilization.[17] In post-obese patients, there is a significant decrease in PDK4 mRNA expression, in conjunction with increased glucose uptake; this is likely due to the downregulation of PDK4 by insulin. This corroborates the concept that a lowered availability of free fatty acids affects glucose metabolism by PDH complex regulation.[18] In fact, it has been shown that insufficient downregulation of PDK mRNA in insulin-resistant individuals could be a cause of increased PDK expression leading to impaired glucose oxidation followed by increased fatty acid oxidation.[19]
Exercise has been shown to induce changes in this gene as well, and that transient changes can have a cumulative effect across many exercise sessions. The mRNA of PDK4, along with PPARGC1A, increases in both types of muscle tissue after exercise.[20][21]
These metabolic effects can be seen in other conditions. Hypoxia is shown to induce PDK4 gene expression through the ERR gamma mechanism.[22] Conversely, PDK4 is downregulated in cardiac muscle tissue during heart failure.[23]
Cancer
[edit]The ubiquitous role of this gene lends itself to being involved in a variety of disease pathologies, including cancer. One metabolite, butyrate, induces hyperacetylation of the histones around the PDK4 gene. This is associated with a greater transcription level of PDK4 mRNA, thereby reversing the downregulation of PDK4 in colon carcinoma cells. In human colon cancer cells, targeting and inactivating the PDH complex limits the metabolic rate and regulates glutamine metabolism, thereby partially inhibiting cell growth.[24] However, PDK4 has also been shown to promote tumor genesis and proliferation through a different pathway, the CREB-RHEB-mTORC1 signaling cascade.[25]
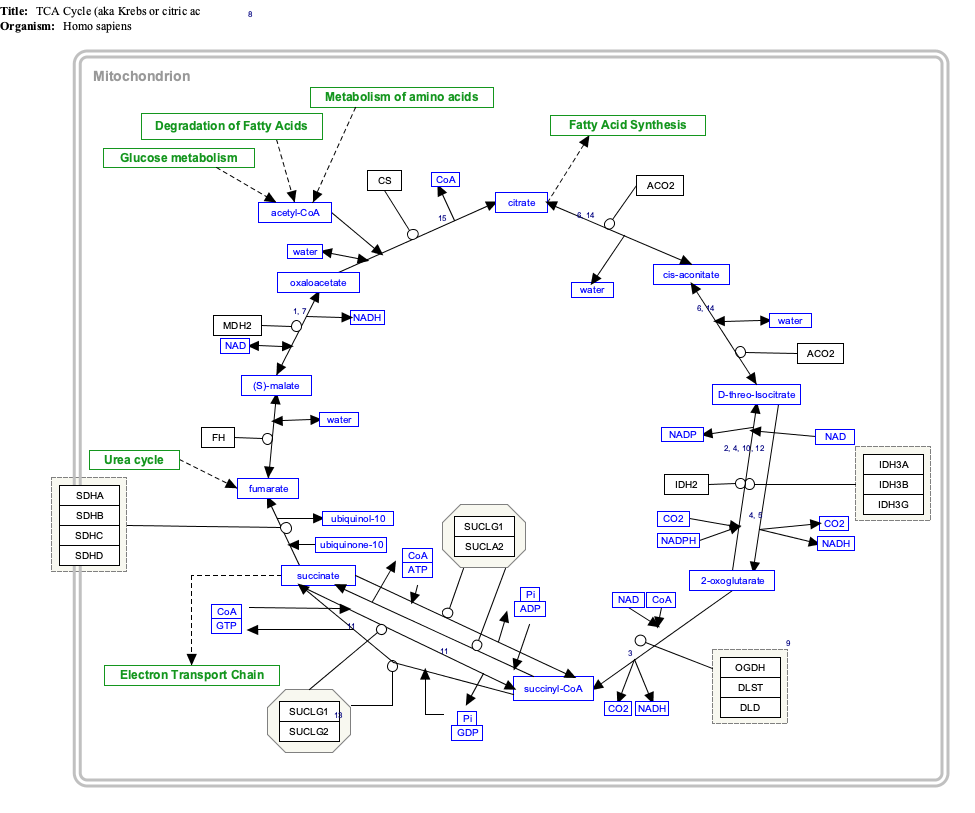
Interactive pathway map
[edit]Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "TCACycle_WP78".
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000004799 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000019577 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Gudi R, Bowker-Kinley MM, Kedishvili NY, Zhao Y, Popov KM (Dec 1995). "Diversity of the pyruvate dehydrogenase kinase gene family in humans". The Journal of Biological Chemistry. 270 (48): 28989–94. doi:10.1074/jbc.270.48.28989. PMID 7499431.
- ^ a b c "Entrez Gene: PDK4 pyruvate dehydrogenase kinase, isozyme 4".
- ^ Andrews MT, Squire TL, Bowen CM, Rollins MB (Jul 1998). "Low-temperature carbon utilization is regulated by novel gene activity in the heart of a hibernating mammal". Proceedings of the National Academy of Sciences of the United States of America. 95 (14): 8392–7. Bibcode:1998PNAS...95.8392A. doi:10.1073/pnas.95.14.8392. PMC 20986. PMID 9653197.
- ^ Kukimoto-Niino M, Tokmakov A, Terada T, Ohbayashi N, Fujimoto T, Gomi S, Shiromizu I, Kawamoto M, Matsusue T, Shirouzu M, Yokoyama S (Sep 2011). "Inhibitor-bound structures of human pyruvate dehydrogenase kinase 4". Acta Crystallographica Section D. 67 (Pt 9): 763–73. doi:10.1107/S090744491102405X. PMID 21904029.
- ^ Kolobova E, Tuganova A, Boulatnikov I, Popov KM (Aug 2001). "Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites". The Biochemical Journal. 358 (Pt 1): 69–77. doi:10.1042/0264-6021:3580069. PMC 1222033. PMID 11485553.
- ^ Kwon HS, Huang B, Ho Jeoung N, Wu P, Steussy CN, Harris RA (2006). "Retinoic acids and trichostatin A (TSA), a histone deacetylase inhibitor, induce human pyruvate dehydrogenase kinase 4 (PDK4) gene expression". Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1759 (3–4): 141–51. doi:10.1016/j.bbaexp.2006.04.005. PMID 16757381.
- ^ Cadoudal T, Distel E, Durant S, Fouque F, Blouin JM, Collinet M, Bortoli S, Forest C, Benelli C (Sep 2008). "Pyruvate dehydrogenase kinase 4: regulation by thiazolidinediones and implication in glyceroneogenesis in adipose tissue". Diabetes. 57 (9): 2272–9. doi:10.2337/db08-0477. PMC 2518477. PMID 18519799.
- ^ Savkur RS, Bramlett KS, Michael LF, Burris TP (Apr 2005). "Regulation of pyruvate dehydrogenase kinase expression by the farnesoid X receptor". Biochemical and Biophysical Research Communications. 329 (1): 391–6. doi:10.1016/j.bbrc.2005.01.141. PMID 15721319.
- ^ Kwon HS, Huang B, Unterman TG, Harris RA (Apr 2004). "Protein kinase B-alpha inhibits human pyruvate dehydrogenase kinase-4 gene induction by dexamethasone through inactivation of FOXO transcription factors". Diabetes. 53 (4): 899–910. doi:10.2337/diabetes.53.4.899. PMID 15047604.
- ^ Abbot EL, McCormack JG, Reynet C, Hassall DG, Buchan KW, Yeaman SJ (Jun 2005). "Diverging regulation of pyruvate dehydrogenase kinase isoform gene expression in cultured human muscle cells". The FEBS Journal. 272 (12): 3004–14. doi:10.1111/j.1742-4658.2005.04713.x. PMID 15955060. S2CID 21366281.
- ^ Spriet LL, Tunstall RJ, Watt MJ, Mehan KA, Hargreaves M, Cameron-Smith D (Jun 2004). "Pyruvate dehydrogenase activation and kinase expression in human skeletal muscle during fasting". Journal of Applied Physiology. 96 (6): 2082–7. doi:10.1152/japplphysiol.01318.2003. PMID 14966024. S2CID 13601849.
- ^ Pilegaard H, Saltin B, Neufer PD (Mar 2003). "Effect of short-term fasting and refeeding on transcriptional regulation of metabolic genes in human skeletal muscle". Diabetes. 52 (3): 657–62. doi:10.2337/diabetes.52.3.657. PMID 12606505.
- ^ Wynn RM, Kato M, Chuang JL, Tso SC, Li J, Chuang DT (Sep 2008). "Pyruvate dehydrogenase kinase-4 structures reveal a metastable open conformation fostering robust core-free basal activity". The Journal of Biological Chemistry. 283 (37): 25305–15. doi:10.1074/jbc.M802249200. PMC 2533096. PMID 18658136.
- ^ Rosa G, Di Rocco P, Manco M, Greco AV, Castagneto M, Vidal H, Mingrone G (Feb 2003). "Reduced PDK4 expression associates with increased insulin sensitivity in postobese patients". Obesity Research. 11 (2): 176–82. doi:10.1038/oby.2003.28. PMID 12582211.
- ^ Majer M, Popov KM, Harris RA, Bogardus C, Prochazka M (Oct 1998). "Insulin downregulates pyruvate dehydrogenase kinase (PDK) mRNA: potential mechanism contributing to increased lipid oxidation in insulin-resistant subjects". Molecular Genetics and Metabolism. 65 (2): 181–6. doi:10.1006/mgme.1998.2748. PMID 9787110.
- ^ Pilegaard H, Ordway GA, Saltin B, Neufer PD (Oct 2000). "Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise". American Journal of Physiology. Endocrinology and Metabolism. 279 (4): E806-14. doi:10.1152/ajpendo.2000.279.4.e806. PMID 11001762. S2CID 1008940.
- ^ Wang L, Sahlin K (Apr 2012). "The effect of continuous and interval exercise on PGC-1α and PDK4 mRNA in type I and type II fibres of human skeletal muscle". Acta Physiologica. 204 (4): 525–32. doi:10.1111/j.1748-1716.2011.02354.x. PMID 21883960. S2CID 13208033.
- ^ Lee JH, Kim EJ, Kim DK, Lee JM, Park SB, Lee IK, Harris RA, Lee MO, Choi HS (2012). "Hypoxia induces PDK4 gene expression through induction of the orphan nuclear receptor ERRγ". PLOS ONE. 7 (9): e46324. Bibcode:2012PLoSO...746324L. doi:10.1371/journal.pone.0046324. PMC 3457976. PMID 23050013.
- ^ Razeghi P, Young ME, Ying J, Depre C, Uray IP, Kolesar J, Shipley GL, Moravec CS, Davies PJ, Frazier OH, Taegtmeyer H (2002). "Downregulation of metabolic gene expression in failing human heart before and after mechanical unloading". Cardiology. 97 (4): 203–9. doi:10.1159/000063122. PMID 12145475. S2CID 46861699.
- ^ Blouin JM, Penot G, Collinet M, Nacfer M, Forest C, Laurent-Puig P, Coumoul X, Barouki R, Benelli C, Bortoli S (Jun 2011). "Butyrate elicits a metabolic switch in human colon cancer cells by targeting the pyruvate dehydrogenase complex". International Journal of Cancer. 128 (11): 2591–601. doi:10.1002/ijc.25599. PMID 20715114. S2CID 27407499.
- ^ Liu Z, Chen X, Wang Y, Peng H, Wang Y, Jing Y, Zhang H (Oct 2014). "PDK4 protein promotes tumorigenesis through activation of cAMP-response element-binding protein (CREB)-Ras homolog enriched in brain (RHEB)-mTORC1 signaling cascade". The Journal of Biological Chemistry. 289 (43): 29739–49. doi:10.1074/jbc.M114.584821. PMC 4207987. PMID 25164809.
Further reading
[edit]- Sugden MC, Holness MJ (May 2003). "Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs". American Journal of Physiology. Endocrinology and Metabolism. 284 (5): E855–62. doi:10.1152/ajpendo.00526.2002. PMID 12676647.
- Kwon HS, Harris RA (2005). "Mechanisms responsible for regulation of pyruvate dehydrogenase kinase 4 gene expression". Advances in Enzyme Regulation. 44: 109–21. doi:10.1016/j.advenzreg.2003.11.020. PMID 15581486.
- Maruyama K, Sugano S (Jan 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Rowles J, Scherer SW, Xi T, Majer M, Nickle DC, Rommens JM, Popov KM, Harris RA, Riebow NL, Xia J, Tsui LC, Bogardus C, Prochazka M (Sep 1996). "Cloning and characterization of PDK4 on 7q21.3 encoding a fourth pyruvate dehydrogenase kinase isoenzyme in human". The Journal of Biological Chemistry. 271 (37): 22376–82. doi:10.1074/jbc.271.37.22376. PMID 8798399.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (Oct 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Majer M, Popov KM, Harris RA, Bogardus C, Prochazka M (Oct 1998). "Insulin downregulates pyruvate dehydrogenase kinase (PDK) mRNA: potential mechanism contributing to increased lipid oxidation in insulin-resistant subjects". Molecular Genetics and Metabolism. 65 (2): 181–6. doi:10.1006/mgme.1998.2748. PMID 9787110.
- Pilegaard H, Ordway GA, Saltin B, Neufer PD (Oct 2000). "Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise". American Journal of Physiology. Endocrinology and Metabolism. 279 (4): E806–14. doi:10.1152/ajpendo.2000.279.4.e806. PMID 11001762. S2CID 1008940.
- Kolobova E, Tuganova A, Boulatnikov I, Popov KM (Aug 2001). "Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites". The Biochemical Journal. 358 (Pt 1): 69–77. doi:10.1042/0264-6021:3580069. PMC 1222033. PMID 11485553.
- Korotchkina LG, Patel MS (Oct 2001). "Site specificity of four pyruvate dehydrogenase kinase isoenzymes toward the three phosphorylation sites of human pyruvate dehydrogenase". The Journal of Biological Chemistry. 276 (40): 37223–9. doi:10.1074/jbc.M103069200. PMID 11486000.
- Peters SJ, Harris RA, Wu P, Pehleman TL, Heigenhauser GJ, Spriet LL (Dec 2001). "Human skeletal muscle PDH kinase activity and isoform expression during a 3-day high-fat/low-carbohydrate diet". American Journal of Physiology. Endocrinology and Metabolism. 281 (6): E1151–8. doi:10.1152/ajpendo.2001.281.6.e1151. PMID 11701428. S2CID 22798857.
- Tuganova A, Boulatnikov I, Popov KM (Aug 2002). "Interaction between the individual isoenzymes of pyruvate dehydrogenase kinase and the inner lipoyl-bearing domain of transacetylase component of pyruvate dehydrogenase complex". The Biochemical Journal. 366 (Pt 1): 129–36. doi:10.1042/BJ20020301. PMC 1222743. PMID 11978179.
- Razeghi P, Young ME, Ying J, Depre C, Uray IP, Kolesar J, Shipley GL, Moravec CS, Davies PJ, Frazier OH, Taegtmeyer H (2002). "Downregulation of metabolic gene expression in failing human heart before and after mechanical unloading". Cardiology. 97 (4): 203–9. doi:10.1159/000063122. PMID 12145475. S2CID 46861699.
- Boulatnikov I, Popov KM (Feb 2003). "Formation of functional heterodimers by isozymes 1 and 2 of pyruvate dehydrogenase kinase". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1645 (2): 183–92. doi:10.1016/S1570-9639(02)00542-3. PMID 12573248.
- Rosa G, Di Rocco P, Manco M, Greco AV, Castagneto M, Vidal H, Mingrone G (Feb 2003). "Reduced PDK4 expression associates with increased insulin sensitivity in postobese patients". Obesity Research. 11 (2): 176–82. doi:10.1038/oby.2003.28. PMID 12582211.
- Pilegaard H, Saltin B, Neufer PD (Mar 2003). "Effect of short-term fasting and refeeding on transcriptional regulation of metabolic genes in human skeletal muscle". Diabetes. 52 (3): 657–62. doi:10.2337/diabetes.52.3.657. PMID 12606505.