Papillary thyroid cancer
| Papillary thyroid cancer | |
|---|---|
 | |
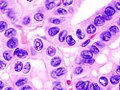
| Cytopathology of papillary thyroid carcinoma, with typical features (Pap stain). | |
| Specialty | ENT surgery |
Papillary thyroid cancer (papillary thyroid carcinoma,[1] PTC) is the most common type of thyroid cancer,[2] representing 75 percent to 85 percent of all thyroid cancer cases.[1] It occurs more frequently in women and presents in the 20–55 year age group. It is also the predominant cancer type in children with thyroid cancer, and in patients with thyroid cancer who have had previous radiation to the head and neck.[3] It is often well-differentiated, slow-growing, and localized, although it can metastasize.
Diagnosis
[edit]Papillary thyroid carcinoma is usually discovered on routine examination as an asymptomatic thyroid nodule that appears as a neck mass. In some instances, the mass may have produced local symptoms. This mass is normally referred to a fine needle aspiration biopsy (FNA) for investigation. FNA accuracy is very high and it is a process widely used in these cases. Other investigation methods include ultrasound imaging and nuclear scan. The ultrasound is a useful test to distinguish solid from cystic lesions and to identify calcifications.[4] The thyroid ultrasound is also very effective to discover microcarcinomas, which refer to very small carcinomas (<1 cm).
Papillary thyroid carcinomas are also discovered when a hard nodule is found in multinodular goiter, when enlarged cervical lymph nodes are detected, or when there are unidentified metastatic lesions elsewhere in the body.[5] Expanding lesions found in the thyroid gland, especially if they are painful, should be examined as they may indicate the presence of papillary thyroid carcinoma. Other clinical signs that could indicate papillary thyroid are fixation to the trachea, a firm neck mass, damage to recurrent laryngeal or cervical sympathetic nerves. Five percent of the population can have thyroid nodules, and the majority will be benign.[6]
Appropriate workup includes an ultrasound of the neck, followed by lab studies. Patients will usually meet with both an endocrinologist and a surgeon (head and neck surgeon or endocrine surgeon).
Markers
[edit]Thyroglobulin can be used as a tumor marker for well-differentiated papillary thyroid cancer.[7][8] HBME-1 staining may be useful for differentiating papillary carcinomas from follicular carcinomas; in papillary lesions it tends to be positive.[9]
Reduced expression of ATP5E is significantly associated with the diagnosis of papillary thyroid cancer and may serve as an early tumor marker of the disease.[10] Serum microRNAs have shown good diagnostic performance for distinguishing patients with papillary thyroid cancer from patients with benign thyroid nodules and healthy controls, and are suggested as novel and minimally invasive diagnostic approach in clinical practice.[11]
Pathology
[edit]


Papillary thyroid cancer gets its name from the papillae among its cells, visible on microscopy. Features include:
- Characteristic Orphan Annie eye nuclear clearings (nuclei with uniform staining, which appear empty due to powdery chromatin and marginal micronucleoli)[12] and psammoma bodies on light microscopy. The former is useful in identifying the follicular variant of papillary thyroid carcinomas.[13]
- Lymphatic spread is more common than hematogenous spread
- Multifocality is common
- The so-called lateral aberrant thyroid is usually a lymph node metastasis from a papillary thyroid carcinoma.[14]
- Papillary microcarcinoma is a subset of papillary thyroid cancer defined as measuring less than or equal to 1 cm.[15] The highest incidence of papillary thyroid microcarcinoma in an autopsy series was reported by Harach et al. in 1985, who found 36 of 101 consecutive autopsies to have an incidental microcarcinoma.[16] Michael Pakdaman et al. report the highest incidence in a retrospective surgical series at 49.9 percent of 860 cases.[17] Management strategies for incidental papillary microcarcinoma on ultrasound (and confirmed on FNAB) range from total thyroidectomy with radioactive iodine ablation to observation alone. Harach et al. suggest using the term "occult papillary tumor" to avoid giving patients distress over having cancer. It was Woolner et al. who first arbitrarily coined the term "occult papillary carcinoma" in 1960, to describe papillary carcinomas ≤ 1.5 cm in diameter.[18]
Several variants are recognized, although classic papillary thyroid carcinoma is the most frequent: microscopic-follicular variant, diffuse-sclerosing variant, tall-cell variant, columnar-cell variant, hobnail variant, and others. The encapsulated-follicular variant, specifically when noninvasive, has been newly reclassified as the noninvasive follicular thyroid neoplasm with papillary-like nuclear features.[19]
Although papillary carcinoma has a propensity to invade lymphatics, it is less likely to invade blood vessels.[20] These kinds of tumors are most commonly unencapsulated, and they have a high tendency to metastasize locally to lymph nodes, which may produce cystic structures near the thyroid that are difficult to diagnose because of the paucity of malignant tissue.[5][21] Furthermore, papillary tumors may metastasize to the lungs and produce a few nodules or the lung fields may exhibit a snowflake appearance throughout.
Other characteristics of the papillary carcinoma is that E.M. shows increased mitochondria, increased RER, as well as increased apical microvilli. Moreover, papillary carcinomas have an indolent growth, and 40 percent of cases spread out of the capsule.[22]
-
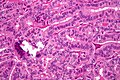
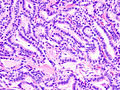
Micrograph of papillary thyroid carcinoma demonstrating prominent papillae with fibrovascular cores. H&E stain.
-
Micrograph showing that the papillae in papillary thyroid carcinoma are composed of cuboidal cells. H&E stain.
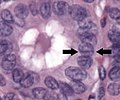
-
Nuclear grooves (arrows indicate one of them)
-
Nuclear pseudoinclusions, which are invaginations of cytoplasm into the nucleus.[23]
-
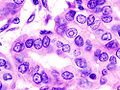
Micrograph (high power view) showing nuclear changes in papillary thyroid carcinoma (PTC), which include groove formation, optical clearing, eosinophilic inclusions and overlapping of nuclei. H&E stain.
-
Micrograph (high power view) of PTC demonstrating nuclear clearing and overlapping nuclei. H&E stain.
-
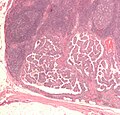
Micrograph of metastatic papillary thyroid carcinoma to a lymph node. H&E stain.
-
Micrograph of papillary thyroid carcinoma, tall cell variant - high magnification. H&E stain.
-
Micrograph of papillary thyroid carcinoma, tall cell variant - intermediate magnification. H&E stain.
Associated mutations
[edit]Mutations associated with papillary thyroid cancer are mainly two forms of chromosomal translocation and one form of point mutation. These alterations lead to activation of a common carcinogenic pathway—the MAPK/ERK pathway.
Chromosomal translocations involving the RET proto-oncogene (encoding a tyrosine kinase receptor that plays essential roles in the development of neuroendocrine cells) located on chromosome 10q11 occur in approximately a fifth of papillary thyroid cancers. The fusion oncoproteins generated are termed RET/PTC proteins (ret/papillary thyroid carcinoma), and constitutively activate RET and the downstream MAPK/ERK pathway.[1] The frequency of ret/PTC translocations is significantly higher in papillary cancers arising in children and after radiation exposure.[1] The gene NTRK1 (encoding the TrkA receptor), located on chromosome 1q, is similarly translocated in approximately 5 percent to 10 percent of papillary thyroid cancers.[1]
Approximately a third to a half of papillary thyroid carcinomas harbor point mutations in the BRAF oncogene, also activating the MAPK/ERK pathway.[1] In those cases the BRAF mutations found were V600E mutation. After performing a multivariate analysis, it was found that the absence of tumor capsule was the only parameter associated (P=0.0005) with BRAF V600E mutation.[5] According to recent studies, papillary cancers carrying the common V600E mutation tend to have a more aggressive long-term course. BRAF mutations are frequent in papillary carcinoma and in undifferentiated cancers that have developed from papillary tumors.
Many more changes in gene expression are currently being investigated. Previous studies demonstrated the dysregulation of different microRNAs in thyroid cancer. For example, downregulation of miR-369-3p and consequent upregulation of its target TSPAN13 appear to be involved in the pathophysiology of PTC.[24]
Mitochondrial mutations: MtDNA(mitochondrial) haplogroups, characterized by unique sets of non pathological mtDNA polymorphisms can modulate the pathogenesis of different diseases in specific populations because of its influence on the expression of genes related to ROS production and OXPHOS coupling efficiency and the regulation of apoptosis.[25] In Asian populations, haplogroup D4a is associated with an increased risk of thyroid cancer[26] while in European populations, Haplogroup K is considered to be protective of Thyroid cancer.[27]
Treatment
[edit]Surgery remains the mainstay of treatment for papillary thyroid cancer. The Revised 2009 American Thyroid Association guidelines for papillary thyroid cancer state that the initial procedure should be near-total or total thyroidectomy. Thyroid lobectomy alone may be sufficient treatment for small (<1 cm), low-risk, unifocal, intrathyroidal papillary carcinomas in the absence of prior head and neck irradiation or radiologically or clinically involved cervical nodal metastasis.[28]
- Minimal disease (diameter up to 1.0 centimeters) - hemithyroidectomy (or unilateral lobectomy) and isthmectomy may be sufficient. There is some discussion whether this is still preferable over total thyroidectomy for this group of patients.
- Gross disease (diameter over 1.0 centimeters) - total thyroidectomy, and central compartment lymph node removal is the therapy of choice. Additional lateral neck nodes can be removed at the same time if an ultrasound guided FNA and thyroglobulin TG cancer washing was positive on the pre-operative neck node ultrasound evaluation.
Arguments for total thyroidectomy are:[29]
- Reduced risk of recurrence, if central compartment nodes are removed at the original surgery.
- 30-85% of papillary carcinoma is multifocal disease. Hemithyroidectomy may leave disease in the other lobe. However, multifocal disease in the remnant lobe may not necessarily become clinically significant or serve as a detriment to patient survival.
- Ease of monitoring with thyroglobulin (sensitivity for picking up recurrence is increased in presence of total thyroidectomy, and ablation of the remnant normal thyroid by low dose radioiodine 131 after following a low iodine diet (LID).
- Ease of detection of metastatic disease by thyroid and neck node ultrasound.
- Post-operative complications at high-volume thyroid surgery centers with experienced surgeons are comparable to that of hemithyroidectomy.
Arguments for hemithyroidectomy:
- Most patients have low-risk cancer with an excellent prognosis, with similar survival outcomes in low-risk patients who undergo total thyroidectomy versus hemithyroidectomy.
- Less likelihood of patient requiring lifelong thyroid hormone replacement after surgery.
Thyroid total body scans are less reliable at finding recurrence than TG and ultrasound.
Papillary tumors tend to be more aggressive in patients over age 45. In such cases, it might be required to perform a more extensive resection including portions of the trachea. Also, the sternocleidomastoid muscle, jugular vein, and accessory nerve are to be removed if such procedure allows apparently complete tumor resection. If a significant amount of residual tumor is left in the neck, external radiotherapy has been indicated and has proven useful especially in those cases when the residual tumor does not take up radioiodine.
After surgical thyroid removal, the patient waits around 4–6 weeks to then have radioiodine therapy. This therapy is intended to both detect and destroy any metastasis and residual tissue in the thyroid. The treatment may be repeated 6–12 months after initial treatment of metastatic disease where disease recurs or has not fully responded.[30]
Patients are administered hormone replacement levothyroxine for life after surgery, especially after total thyroidectomy. Chemotherapy with cisplatin or doxorubicin has proven limited efficacy, however, it could be helpful for patients with bone metastases to improve their quality of life. Patients are also prescribed levothyroxine and radioiodine after surgery. Levothyroxine influences growth and maturation of tissues and it is involved in normal growth, metabolism, and development. In case of metastases, patients are prescribed antineoplastic agents which inhibit cell growth and proliferation and help in palliating symptoms in progressive disease.
After successful treatment, 35 percent of the patients may experience a recurrence within a 40-year span. Also, patients may experience a high incidence of nodule metastasis, with 35 percent cases of cervical node metastases. Approximately 20 percent of patients will develop multiple tumors within the thyroid gland.[31]
There is ongoing discussion regarding the best management regarding the optimal surgical procedure for papillary thyroid cancer. Prognosis of patients with papillary thyroid cancer is found to be dependent on the patient's age, the size of the tumor, presence of metastatic disease, and the presence of tumor invasion into adjacent tissues near the thyroid gland. Recent studies have examined a more conservative approach to surgery and have demonstrated that hemithyroidectomy may be acceptable for patients with low-risk papillary thyroid cancer with tumor size 1 cm to 4 cm with no presence of invasion to tissues surrounding the thyroid or metastasis. Studies examining large databases of patients with papillary thyroid cancer have concluded that there is no survival advantage for patients with stage I papillary thyroid cancer size 1–4 cm receiving total thyroidectomy versus hemithyroidectomy.[32] In light of this data, choosing the optimal course of surgical and medical management of papillary thyroid cancer should involve shared decision making from patient, endocrinologists, and surgeons.
Prognosis
[edit]Depending on source, the overall 5-year survival rate for papillary thyroid cancer is 96 percent[33] or 97 percent,[20] with a 10-year survival rate of 93 percent.[33]
For a more specific prognosis for individual cases, there are at minimum 13 known scoring systems for prognosis; among the more often used are:
- AGES - Age, Grade, Extent of disease, Size
- AMES - Age, Metastasis, Extent of disease, Size
- MACIS - Metastasis, Age at presentation, Completeness of surgical resection, Invasion (extrathyroidal), Size[34] (this is a modification of the AGES system). It is probably the most reliable staging method available. Also known as the MAICS system.
- TNM staging - Tumor, node, metastasis. Remarkable about the TNM staging for (differentiated) thyroid carcinoma is that the scoring is different according to age.
MACIS
[edit]The MACIS system of estimating the prognosis of papillary thyroid cancer was developed by Clive S. Grant at the Mayo Clinic and was based on careful evaluation of a large group of patients. It is probably the most reliable staging method available.[35]
It assigns scores to the main factors involved and uses the sum of this score to calculate the prognosis:
| Factors[35] | Score[35] | |
|---|---|---|
| Distant Metastasis: spread of the cancer to areas outside the neck | Yes | 3 |
| No | 0 | |
| Age at the time the tumor was discovered | Less than 39 years | 3.1 |
| Over 40 years | 0.08 x age | |
| Invasion into surrounding areas of the neck as seen by the naked eye | Yes | 1 |
| No | 0 | |
| Completeness of surgical resection (or removal) of the tumor | Incomplete | 1 |
| Complete | 0 | |
| Size of the tumor | 0.3 x size in cm | |
| Sum of MACIS score[35] | 20 yr Survival[35] |
|---|---|
| < 6.0 | 99% |
| 6.0 - 6.99 | 89% |
| 7.0 - 7.99 | 56% |
| > 8.0 | 24% |
Most patients fall into the low-risk category (MACIS score less than 6.0) and are cured of the cancer at the time of surgery.[35]
Children with multiple lung metastases and/or a miliary aspect still have an excellent long-term prognosis if given adequate treatment.[36]
Stage
[edit]Based on overall cancer staging into stages I to IV, papillary thyroid cancer has a 5-year survival rate of 100 percent for stages I and II, 93 percent for stage III and 51 percent for stage IV.[37]
Epidemiology
[edit]
According to Surveillance, Epidemiology, and End Results (SEER), the incidence of papillary cancer has increased from 4.8 to 14.9 per 100,000 from 1975 to 2012. Females are more likely to get papillary cancer when compared to males with incidence ratio of 2.5 to 1 where most of the cancers are diagnosed between 40 and 50 years old in females. However, death rates from papillary cancer remains static from 2003 to 2012 at 0.5 per 100,000 men and women. There was an increased incidence of papillary cancer from 1910 to 1960 due to the use of ionising radiation in treating childhood head and neck cancers.[39] The incidence decreased after radiation therapy was abandoned. Environmental exposures to radiation such as atomic bombings of Hiroshima and Nagasaki and Chernobyl disaster also causes an increase in childhood papillary thyroid cancer at 5 to 20 years after the exposure to radiation.[40] Family history of thyroid cancer syndrome such as familial adenomatous polyposis, Carney complex, Multiple endocrine neoplasia type 2 (MEN-2), Werner syndrome, and Cowden syndrome increases the risk of getting papillary cancer.[39]
References
[edit]- ^ a b c d e f Chapter 20 in: Mitchell, Richard Sheppard; Kumar, Vinay; Abbas, Abul K; Fausto, Nelson (2007). Robbins Basic Pathology. Philadelphia: Saunders. ISBN 978-1-4160-2973-1. 8th edition.
- ^ Hu MI, Vassilopoulou-Sellin R, Lustig R, Lamont JP "Thyroid and Parathyroid Cancers" Archived 2010-02-28 at the Wayback Machine in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds) Cancer Management: A Multidisciplinary Approach. Archived 2013-10-04 at the Wayback Machine 11 ed. 2008.
- ^ Dinets A, Hulchiy M, Sofiadis A, Ghaderi M, Höög A, Larsson C, Zedenius J (June 2012). "Clinical, genetic, and immunohistochemical characterization of 70 Ukrainian adult cases with post-Chornobyl papillary thyroid carcinoma". European Journal of Endocrinology. 166 (6): 1049–1060. doi:10.1530/EJE-12-0144. PMC 3361791. PMID 22457234.
- ^ Al-Brahim N, Asa SL (July 2006). "Papillary thyroid carcinoma: an overview". Archives of Pathology & Laboratory Medicine. 130 (7): 1057–1062. doi:10.5858/2006-130-1057-PTCAO. PMID 16831036.
- ^ a b c "The Thyroid and its Diseases". Archived from the original on 2010-07-01. Retrieved 2010-07-15.
- ^ Surgical Recall 7th Edition ISBN 978-1451192919[page needed]
- ^ Lin JD (February 2008). "Thyroglobulin and human thyroid cancer". Clinica Chimica Acta; International Journal of Clinical Chemistry. 388 (1–2): 15–21. doi:10.1016/j.cca.2007.11.002. PMID 18060877.
- ^ Tuttle RM, Leboeuf R, Martorella AJ (September 2007). "Papillary thyroid cancer: monitoring and therapy". Endocrinology and Metabolism Clinics of North America. 36 (3): 753–78, vii. doi:10.1016/j.ecl.2007.04.004. PMID 17673127.
- ^ Papotti M, Rodriguez J, De Pompa R, Bartolazzi A, Rosai J (April 2005). "Galectin-3 and HBME-1 expression in well-differentiated thyroid tumors with follicular architecture of uncertain malignant potential". Modern Pathology. 18 (4): 541–546. doi:10.1038/modpathol.3800321. PMID 15529186.
- ^ Hurtado-López LM, Fernández-Ramírez F, Martínez-Peñafiel E, Carrillo Ruiz JD, Herrera González NE (June 2015). "Molecular Analysis by Gene Expression of Mitochondrial ATPase Subunits in Papillary Thyroid Cancer: Is ATP5E Transcript a Possible Early Tumor Marker?". Medical Science Monitor. 21: 1745–1751. doi:10.12659/MSM.893597. PMC 4482184. PMID 26079849.
- ^ Chen, Yuping; Dong, Bingtian; Huang, Lichun; Huang, Huibin (2022-06-10). "Serum microRNAs as biomarkers for the diagnosis of papillary thyroid carcinoma: a meta-analysis". Bosnian Journal of Basic Medical Sciences. 22 (6): 862–871. doi:10.17305/bjbms.2022.7343. ISSN 1840-4812. PMC 9589316. PMID 35678022.
- ^ "Papillary Carcinoma of Thyroid (Hi Pow)". University of Connecticut Health Center. Archived from the original on 2012-07-09. Retrieved 2008-09-14.
- ^ Yang GC, Liebeskind D, Messina AV (June 2001). "Ultrasound-guided fine-needle aspiration of the thyroid assessed by Ultrafast Papanicolaou stain: data from 1135 biopsies with a two- to six-year follow-up". Thyroid. 11 (6): 581–589. doi:10.1089/105072501750302895. PMID 11442006.
- ^ Escofet X, Khan AZ, Mazarani W, Woods WG (January 2007). "Lessons to be learned: a case study approach. Lateral aberrant thyroid tissue: is it always malignant?". The Journal of the Royal Society for the Promotion of Health. 127 (1): 45–46. doi:10.1177/1466424007073207. PMID 17319317. S2CID 33217834.
- ^ Shaha AR (May 2007). "TNM classification of thyroid carcinoma". World Journal of Surgery. 31 (5): 879–887. doi:10.1007/s00268-006-0864-0. PMID 17308849. S2CID 23121159.
- ^ Harach HR, Franssila KO, Wasenius VM (August 1985). "Occult papillary carcinoma of the thyroid. A "normal" finding in Finland. A systematic autopsy study". Cancer. 56 (3): 531–538. doi:10.1002/1097-0142(19850801)56:3<531::AID-CNCR2820560321>3.0.CO;2-3. PMID 2408737. S2CID 23922855.
- ^ Pakdaman MN, Rochon L, Gologan O, Tamilia M, Garfield N, Hier MP, et al. (November 2008). "Incidence and histopathological behavior of papillary microcarcinomas: study of 429 cases". Otolaryngology–Head and Neck Surgery. 139 (5): 718–722. doi:10.1016/j.otohns.2008.08.014. PMID 18984270. S2CID 5937993.
- ^ Woolner LB, Lemmon ML, Beahrs OH, Black BM, Keating FR (January 1960). "Occult papillary carcinoma of the thyroid gland: a study of 140 cases observed in a 30-year period". The Journal of Clinical Endocrinology and Metabolism. 20 (1): 89–105. doi:10.1210/jcem-20-1-89. PMID 13845950.
- ^ Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. (August 2016). "Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors". JAMA Oncology. 2 (8): 1023–1029. doi:10.1001/jamaoncol.2016.0386. PMC 5539411. PMID 27078145.
- ^ a b Papillary Thyroid Carcinoma at eMedicine
- ^ Grani G, Fumarola A (June 2014). "Thyroglobulin in lymph node fine-needle aspiration washout: a systematic review and meta-analysis of diagnostic accuracy". The Journal of Clinical Endocrinology and Metabolism. 99 (6): 1970–1982. doi:10.1210/jc.2014-1098. PMID 24617715.
- ^ "Papillary Carcinomas". Archived from the original on April 19, 2010. Retrieved 2010-07-15.
- ^ Ip YT, Dias Filho MA, Chan JK (December 2010). "Nuclear inclusions and pseudoinclusions: friends or foes of the surgical pathologist?". International Journal of Surgical Pathology. 18 (6): 465–481. doi:10.1177/1066896910385342. PMID 21081532. S2CID 22429137.
- ^ Li P, Dong M, Wang Z (May 2019). "Downregulation of TSPAN13 by miR-369-3p inhibits cell proliferation in papillary thyroid cancer (PTC)". Bosnian Journal of Basic Medical Sciences. 19 (2): 146–154. doi:10.17305/bjbms.2018.2865. PMC 6535391. PMID 30114378.
- ^ Bai RK, Leal SM, Covarrubias D, Liu A, Wong LJ (May 2007). "Mitochondrial genetic background modifies breast cancer risk". Cancer Research. 67 (10): 4687–4694. doi:10.1158/0008-5472.CAN-06-3554. PMID 17510395.
- ^ Fang H, Shen L, Chen T, He J, Ding Z, Wei J, et al. (August 2010). "Cancer type-specific modulation of mitochondrial haplogroups in breast, colorectal and thyroid cancer". BMC Cancer. 10 (1): 421. doi:10.1186/1471-2407-10-421. PMC 2933623. PMID 20704735.
- ^ Cocoş R, Schipor S, Badiu C, Raicu F (March 2018). "Mitochondrial DNA haplogroup K as a contributor to protection against thyroid cancer in a population from southeast Europe". Mitochondrion. 39: 43–50. doi:10.1016/j.mito.2017.08.012. PMID 28851673.
- ^ Cooper DS, Doherty GM, Haugen BR, Hauger BR, Kloos RT, Lee SL, et al. (November 2009). "Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer". Thyroid. 19 (11): 1167–1214. doi:10.1089/thy.2009.0110. hdl:2027.42/78131. PMID 19860577.
- ^ Udelsman R, Shaha AR (July 2005). "Is total thyroidectomy the best possible surgical management for well-differentiated thyroid cancer?". The Lancet. Oncology. 6 (7): 529–531. doi:10.1016/S1470-2045(05)70247-3. PMID 15992702.
- ^ Papillary Thyroid Carcinoma~treatment at eMedicine
- ^ "Papillary Thyroid Carcinoma". Archived from the original on July 19, 2008. Retrieved 2010-07-15.
- ^ Adam MA, Pura J, Goffredo P, Dinan MA, Hyslop T, Reed SD, et al. (January 2015). "Impact of extent of surgery on survival for papillary thyroid cancer patients younger than 45 years". The Journal of Clinical Endocrinology and Metabolism. 100 (1): 115–121. doi:10.1210/jc.2014-3039. PMC 5399499. PMID 25337927.
- ^ a b Numbers from National Cancer Database in the US, from Page 10 in: Biersack, H-J; Grünwald, F, eds. (2005). Thyroid Cancer. Berlin: Springer. ISBN 978-3-540-22309-2. (Note: Book also states that the 14 percent 10-year survival for anaplastic thyroid cancer was overestimated)
- ^ "New York Thyroid Center: Prognosis Staging for Thyroid Cancer". Archived from the original on 2007-12-14. Retrieved 2007-12-22.
- ^ a b c d e f New York Thyroid Center > Thyroid cancer > Prognosis staging Retrieved on April 30, 2010
- ^ Vermeer-Mens JC, Goemaere NN, Kuenen-Boumeester V, de Muinck Keizer-Schrama SM, Zwaan CM, Devos AS, de Krijger RR (December 2006). "Childhood papillary thyroid carcinoma with miliary pulmonary metastases". Journal of Clinical Oncology. 24 (36): 5788–5789. doi:10.1200/JCO.2006.08.8732. PMID 17179115.
- ^ cancer.org > Thyroid Cancer By the American Cancer Society. In turn citing: AJCC Cancer Staging Manual (7th ed).
- ^ Andrey Bychkov, M.D., Ph.D. "Thyroid & parathyroid - Congenital / metabolic anomalies - Thyroglossal duct cyst". PathologyOutlines.
{{cite web}}: CS1 maint: multiple names: authors list (link) Topic Completed: 14 March 2016. Minor changes: 27 January 2021. - ^ a b Tuttle RM, Ross DS, Mulder JE. "Papillary thyroid cancer". UpToDate. Retrieved 13 October 2017.
- ^ Vaisman F, Corbo R, Vaisman M (2011). "Thyroid carcinoma in children and adolescents-systematic review of the literature". Journal of Thyroid Research. 2011: 845362. doi:10.4061/2011/845362. PMC 3166725. PMID 21904689.
External links
[edit]- Thyroid cancer at DMOZ
- Cancer Management Handbook: Thyroid and Parathyroid Cancers
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. (January 2016). "2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer". Thyroid. 26 (1): 1–133. doi:10.1089/thy.2015.0020. PMC 4739132. PMID 26462967.


![Nuclear pseudoinclusions, which are invaginations of cytoplasm into the nucleus.[23]](http://178.128.105.246/host-http-upload.wikimedia.org/wikipedia/commons/thumb/9/9d/Histopathology_of_papillary_thyroid_cancer_in_a_thyroglossal_cyst%2C_high_magnification%2C_annotated.jpg/120px-Histopathology_of_papillary_thyroid_cancer_in_a_thyroglossal_cyst%2C_high_magnification%2C_annotated.jpg)