Periodate
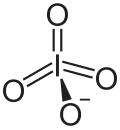 The metaperiodate ion
| |
 The orthoperiodate ion
| |
| Names | |
|---|---|
| Systematic IUPAC name
tetraoxoiodate(1−) hexaoxoiodate(5−) | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| IO4− or IO65- | |
| Conjugate acid | Periodic acid |
| Related compounds | |
Other anions
|
Perchlorate Perbromate Permanganate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Periodate (/pəˈraɪ.ədeɪt/ pə-RY-ə-dayt) is an anion composed of iodine and oxygen. It is one of a number of oxyanions of iodine and is the highest in the series, with iodine existing in oxidation state +7. Unlike other perhalogenates, such as perchlorate, it can exist in two forms: metaperiodate IO−
4 and orthoperiodate IO5−
6. In this regard it is comparable to the tellurate ion from the adjacent group. It can combine with a number of counter ions to form periodates, which may also be regarded as the salts of periodic acid.
Periodates were discovered by Heinrich Gustav Magnus and C. F. Ammermüller; who first synthesised periodic acid in 1833.[1]
Synthesis
[edit]Classically, periodate was most commonly produced in the form of sodium hydrogen periodate (Na3H2IO6).[2] This is commercially available, but can also be produced by the oxidation of iodates with chlorine and sodium hydroxide.[3] Or, similarly, from iodides by oxidation with bromine and sodium hydroxide:
Modern industrial scale production involves the electrochemical oxidation of iodates, on a lead dioxide (PbO2) anode, with the following standard electrode potential:
Metaperiodates are typically prepared by the dehydration of sodium hydrogen periodate with nitric acid,[2] or by dehydrating orthoperiodic acid by heating it to 100 °C under vacuum.
They can also be generated directly from iodates by treatment with other strong oxidizing agents such as hypochlorites:
Forms and interconversion
[edit]Periodate can exist in a variety of forms in aqueous media, with pH being a controlling factor. Orthoperiodate has a number of acid dissociation constants.[5][6]
- pKa = 3.29
- pKa = 8.31
- pKa = 11.60
The ortho- and metaperiodate forms also exist in equilibrium.
- K = 29
For this reason orthoperiodate is sometimes referred to as the dihydrate of metaperiodate,[7] written IO−4·2H2O; however, this description is not strictly accurate as X-ray crystallography of H5IO6 shows 5 equivalent I−OH groups.[8]
At extremes of pH additional species can form. Under basic conditions a dehydration reaction can take place to form the diperiodate (sometimes referred to as mesoperiodate).
- K = 820
Under strongly acid conditions periodic acid can be protonated to give the orthoperiodonium cation.[9]
- pKa = −0.8
Structure and bonding
[edit]In both the ortho- and metaperiodate the iodine is hypervalent, as it forms more bonds than would classically be allowed. This has been explained in terms of dative bonds, confirming the absence of double bonding in these molecules.[10]
Exact structures vary depending on counter ions, however on average orthoperiodates adopt a slightly deformed octahedral geometry with X-ray diffraction showing I–O bond lengths of 1.89 Å.[11][8] Metaperiodates adopt a distorted tetrahedral geometry with an average I–O distance of 1.78 Å.[12][13]
Reactions
[edit]Cleavage reactions
[edit]Periodates can cleave carbon–carbon bonds on a variety of 1,2-difunctionalised alkanes.[14][15] The most common example of this is diol cleavage, which was also the first to be discovered (Malaprade reaction).[16] In addition to diols, periodates can cleave 1,2-hydroxy ketones, 1,2-diketones, α-keto acids, α-hydroxy acids, amino acids,[17] 1,2-amino alcohols,[18] and 1,2-diamines,[19] to give aldehydes, ketones, and carboxylic acids. In the presence of strong acid catalyst, like H2SO4 or HNO3 epoxides[20][21] are also converted into aldehyde or ketones or dicarbonyl compounds.

Alkenes can also be oxidised and cleaved in the Lemieux–Johnson oxidation. This uses a catalytic loading of osmium tetroxide which is regenerated in situ by the periodate. The overall process is equivalent to that of ozonolysis.

Cleavage reactions proceed via a cyclic intermediate called a periodate ester. The formation of this may be affected by pH and temperature[22] but is most strongly affected by the geometry of the substrate, with cis-diols reacting significantly faster than trans-diols.[23] The reactions are exothermic and are typically performed at 0 °C. As periodate salts are only readily soluble in water reactions are generally performed in aqueous media. Where solubility is an issue periodic acid may be used, as this is soluble in alcohols; phase transfer catalysts are also effective in biphasic reaction mixtures. In extreme cases the periodate may be exchanged for lead tetraacetate which reacts in a similar manner and is soluble in organic solvents (Criegee oxidation).

Periodate cleavage is often utilized in molecular biochemistry for the purposes of modifying saccharide rings, as many five- and six-membered sugars have vicinal diols. Historically it was also used to determine the structure of monosaccharides.[24][25]
Periodate cleavage may be performed on an industrial scale to form dialdehyde starch which has uses in paper production.[26]
Oxidation reactions
[edit]Periodates are powerful oxidising agents. They can oxidise catechol to 1,2-benzoquinone and hydroquinone to 1,4-benzoquinone.[27] Sulfides can be effectively oxidised to sulfoxides.[28] Periodates are sufficiently powerful to generate other strong inorganic oxidisers such as permanganate,[29] osmium tetroxide[30] and ruthenium tetroxide.
Niche uses
[edit]Periodates are highly selective etchants for certain ruthenium-based oxides.[31]
Several staining agents use in microscopy are based around periodate (e.g. periodic acid–Schiff stain and Jones' stain).
Periodates have also been used as oxidising agents for use in pyrotechnics.[32] In 2013 the US Army announced that it would replace the environmentally harmful chemicals barium nitrate and potassium perchlorate with sodium metaperiodate for use in their tracer ammunition.[33]
Other oxyanions
[edit]Periodate is part of a series of oxyanions in which iodine can assume oxidation states of −1, +1, +3, +5, or +7. A number of neutral iodine oxides are also known.
| Iodine oxidation state | −1 | +1 | +3 | +5 | +7 |
|---|---|---|---|---|---|
| Name | iodide | hypoiodite | iodite | iodate | periodate |
| Formula | I− | IO− | IO− 2 |
IO− 3 |
IO− 4 or IO5− 6 |
| Structure | 
|
See also
[edit]References
[edit]- ^ Ammermüller, F.; Magnus, G. (1833). "Ueber eine neue Verbindung des Jods mit Sauerstoff, die Ueberjodsäure". Annalen der Physik und Chemie (in German). 104 (7): 514–525. Bibcode:1833AnP...104..514A. doi:10.1002/andp.18331040709.
- ^ a b Schmeisser, M. (1963). Georg Brauer. (ed.). Handbook of Preparative Inorganic Chemistry. Vol. 1 (2nd ed.). New York, N.Y.: Academic Press. pp. 323–324. ISBN 012126601X.
- ^ Hill, Arthur E. (October 1928). "Ternary Systems. VII. The Periodates of the Alkali Metals". Journal of the American Chemical Society. 50 (10): 2678–2692. doi:10.1021/ja01397a013.
- ^ Parsons, Roger (1959). Handbook of electrochemical constants. Butterworths Scientific Publications Ltd. p. 71.
- ^ Aylett, founded by A.F. Holleman; continued by Egon Wiberg; translated by Mary Eagleson, William Brewer; revised by Bernhard J. (2001). Inorganic chemistry (1st English ed., [edited] by Nils Wiberg. ed.). San Diego, Calif.: Berlin: Academic Press, W. de Gruyter. p. 454. ISBN 0123526515.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Burgot, Jean-Louis (30 March 2012). Ionic equilibria in analytical chemistry. New York: Springer. p. 358. ISBN 978-1441983824.
- ^ Ropp, Richard C. (31 December 2012). Encyclopedia of the alkaline earth compounds. Oxford: Elsevier Science. p. 96. ISBN 978-0444595539.
- ^ a b Feikema, Y. D. (1966). "The crystal structures of two oxy-acids of iodine. I. A study of orthoperiodic acid, H5IO6, by neutron diffraction". Acta Crystallographica. 20 (6): 765–769. doi:10.1107/S0365110X66001828.
- ^ Greenwood, N.N.; Earnshaw, A. (2006). Chemistry of the elements (2nd ed.). Oxford: Butterworth-Heinemann. p. 874. ISBN 0750633654.
- ^ Ivanov, A.; Popov, A.; Boldyrev, A.; Zhdankin, V. (2014). "The I=X (X = O,N,C) Double Bond in Hypervalent Iodine Compounds: Is it Real?". Angew. Chem. Int. Ed. 53 (36): 9617–9621. doi:10.1002/anie.201405142. PMID 25045143.
- ^ Tichý, K.; Rüegg, A.; Beneš, J. (1980). "Neutron diffraction study of diammonium trihydrogen periodate, (NH4)2H3IO6, and its deuterium analogue, (ND4)2D3IO6". Acta Crystallographica Section B. 36 (5): 1028–1032. doi:10.1107/S0567740880005225.
- ^ Levason, W.; Webster, M. (15 June 1999). "Ammonium tetraoxoiodate(VII)". Acta Crystallographica Section C. 55 (6): IUC9900052. doi:10.1107/S0108270199099394.
- ^ Kálmán, A.; Cruickshank, D. W. J. (1970). "Refinement of the structure of NaIO4". Acta Crystallographica Section B. 26 (11): 1782–1785. doi:10.1107/S0567740870004880.
- ^ Sklarz, B. (1967). "Organic chemistry of periodates". Quarterly Reviews, Chemical Society. 21 (1): 3. doi:10.1039/QR9672100003.
- ^ Bamford, C. H., ed. (1972). Reactions of non-metallic inorganic compounds. Amsterdam: Elsevier Pub. Co. p. 435. ISBN 9780080868011.
- ^ L. Malaprade, Bull. Soc. Chim. Fr. 3, 1, 833 (1934)
- ^ Clamp, J.R.; Hough, L. (Jan 1965). "The Periodate Oxidation of Amino Acids with Reference to Studies on Glycoproteins". The Biochemical Journal. 94 (1): 17–24. doi:10.1042/bj0940017. PMC 1206400. PMID 14342227.
- ^ Nicolet, Ben H.; Shinn, Leo A. (June 1939). "THE ACTION OF PERIODIC ACID ON α-AMINO ALCOHOLS". Journal of the American Chemical Society. 61 (6): 1615. doi:10.1021/ja01875a521.
- ^ Maros, László; Molnár-Perl, Ibolya; Schissel, Enikó; Szerdahelyi, Vilmos (1980). "Mechanism of the periodate oxidation of ethane-1,2-diamine, N,N′-dimethylethane-1,2-diamine, and 2-aminoethanol". Journal of the Chemical Society, Perkin Transactions 2 (1): 39–45. doi:10.1039/P29800000039.
- ^ Telvekar, Vikas N.; Patel, Dharmeshkumar J.; Mishra, Sanket J. (2008). "Oxidative Cleavage of Epoxides Using Aqueous Sodium Paraperiodate". Synthetic Communications. 39 (2): 311–315. doi:10.1080/00397910802372574. S2CID 97403497.
- ^ Richard, Fuchs; Waters, R. C.; Vanderwerf C. A. (1952). "General Qualitative Test for Epoxides". Analytical Chemistry. 24 (9): 1514. doi:10.1021/ac60069a047.
- ^ Buist, G. J.; Bunton, C. A.; Hipperson, W. C. P. (1971). "The mechanism of oxidation of α-glycols by periodic acid. Part X. The oxidation of pinacol, and a general discussion of the stability of periodate esters and their role in the mechanism of oxidation". Journal of the Chemical Society B: Physical Organic: 2128–2142. doi:10.1039/J29710002128.
- ^ McMurry, John (2012). Organic chemistry (8th ed., [international ed.] ed.). Singapore: Brooks/Cole Cengage Learning. p. 312. ISBN 978-0840054531.
- ^ Jackson, Ernest L.; Hudson, C. S. (June 1937). "Studies on the Cleavage of the Carbon Chain of Glycosides by Oxidation. A New Method for Determining Ring Structures and Alpha and Beta Configurations of Glycosides". Journal of the American Chemical Society. 59 (6): 994–1003. doi:10.1021/ja01285a010.
- ^ Robyt, John F. (1998). Essentials of carbohydrate chemistry. New York: Springer. ISBN 0387949518.
- ^ Yu, Jiugao; Chang, Peter R.; Ma, Xiaofei (January 2010). "The preparation and properties of dialdehyde starch and thermoplastic dialdehyde starch". Carbohydrate Polymers. 79 (2): 296–300. doi:10.1016/j.carbpol.2009.08.005.
- ^ Weidman, S. W.; Kaiser, E. T. (December 1966). "The Mechanism of the Periodate Oxidation of Aromatic Systems. III. A Kinetic Study of the Periodate Oxidation of Catechol". Journal of the American Chemical Society. 88 (24): 5820–5827. doi:10.1021/ja00976a024.
- ^ Leonard, Nelson J.; Johnson, Carl R. (January 1962). "Periodate Oxidation of Sulfides to Sulfoxides. Scope of the Reaction". The Journal of Organic Chemistry. 27 (1): 282–284. doi:10.1021/jo01048a504.
- ^ Lemieux, R. U.; Rudloff, E. Von (November 1955). "Periodate–Permanganate Oxidations: I. Oxidation of Olefins". Canadian Journal of Chemistry. 33 (11): 1701–1709. doi:10.1139/v55-208.
- ^ Pappo, R.; Allen, D. S. Jr.; Lemieux, R. U.; Johnson, W. S. (1956). "Notes - Osmium Tetroxide-Catalyzed Periodate Oxidation of Olefinic Bonds". The Journal of Organic Chemistry. 21 (4): 478–479. doi:10.1021/jo01110a606. ISSN 0022-3263.
- ^ Dieter Weber, Róza Vöfély, Yuehua Chen, Yulia Mourzina, Ulrich Poppe: Variable resistor made by repeated steps of epitaxial deposition and lithographic structuring of oxide layers by using wet chemical etchants. Thin Solid Films (2013) DOI: 10.1016/j.tsf.2012.11.118
- ^ Moretti, Jared D.; Sabatini, Jesse J.; Chen, Gary (9 July 2012). "Periodate Salts as Pyrotechnic Oxidizers: Development of Barium- and Perchlorate-Free Incendiary Formulations". Angewandte Chemie International Edition. 51 (28): 6981–6983. doi:10.1002/anie.201202589. PMID 22639415.
- ^ "Picatinny to remove tons of toxins from lethal rounds". U.S. Army. Retrieved 31 October 2013.












