tert-Butyllithium

| |
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
tert-Butyllithium[citation needed] | |
| Identifiers | |
3D model (JSmol)
|
|
| 3587204 | |
| ChemSpider | |
| ECHA InfoCard | 100.008.939 |
| EC Number |
|
PubChem CID
|
|
| UN number | 3394 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| LiC 4H 9 | |
| Molar mass | 64.055 g mol−1 |
| Appearance | Colorless solid |
| Density | 660 mg cm−3 |
| Boiling point | 36 to 40 °C (97 to 104 °F; 309 to 313 K) |
| Reacts | |
| Acidity (pKa) | 45–53 |
| Hazards | |
| GHS labelling: | |
    
| |
| Danger | |
| H225, H250, H260, H300, H304, H310, H314, H330, H336, H411 | |
| P210, P222, P223, P231+P232, P370+P378, P422 | |
| NFPA 704 (fire diamond) | |
| Flash point | −6.6 °C (20.1 °F; 266.5 K) |
| Related compounds | |
Related compounds
|
n-Butyllithium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
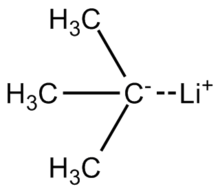
tert-Butyllithium is a chemical compound with the formula (CH3)3CLi. As an organolithium compound, it has applications in organic synthesis since it is a strong base, capable of deprotonating many carbon molecules, including benzene. tert-Butyllithium is available commercially as solutions in hydrocarbons (such as pentane); it is not usually prepared in the laboratory.
Preparation
[edit]tert-Butyllithium is produced commercially by treating tert-butyl chloride with lithium metal. Its synthesis was first reported by R. B. Woodward in 1941.[1]
Structure and bonding
[edit]Like other organolithium compounds, tert-butyllithium is a cluster compound. Whereas n-butyllithium exists both as a hexamer and a tetramer, tert-butyllithium exists exclusively as a tetramer with a cubane structure. Bonding in organolithium clusters involves sigma delocalization and significant Li−Li bonding.[2] Despite its complicated structure, tert-butyllithium is usually depicted in equations as a monomer.
The lithium–carbon bond in tert-butyllithium is highly polarized, having about 40 percent ionic character. The molecule reacts like a carbanion, as is represented by these two resonance structures:[3]
Reactions
[edit]tert-Butyllithium is renowned for deprotonation of carbon acids (C-H bonds). One example is the double deprotonation of allyl alcohol.[4] Other examples are the deprotonation of vinyl ethers.[5][6][7]
In combination with n-butyllithiium, tert-butylllithium monolithiates ferrocene.[8] tert-Butyllithium deprotonates dichloromethane:[9]
- H2CCl2 + RLi → HCCl2Li + RH
Similar to n-butyllithium, tert-butyllithium can be used for lithium–halogen exchange reactions.[10][11]
Solvent compatibility
[edit]To minimize degradation by solvents, reactions involving tert-butyllithium are often conducted at very low temperatures in special solvents, such as the Trapp solvent mixture.
More so than other alkyllithium compounds, tert-butyllithium reacts with ethers.[2] In diethyl ether, the half-life of tert-butyllithium is about 60 minutes at 0 °C. It is even more reactive toward tetrahydrofuran (THF); the half-life in THF solutions is about 40 minutes at −20 °C.[12] In dimethoxyethane, the half-life is about 11 minutes at −70 °C[13]
In this example, the reaction of tert-butyllithium with (THF) is shown:
Safety
[edit]tert-butyllithium is a pyrophoric substance, meaning that it spontaneously ignites on exposure to air. Air-free techniques are important so as to prevent this compound from reacting violently with oxygen and moisture:
- t-BuLi + O2 → t-BuOOLi
- t-BuLi + H2O → t-BuH + LiOH
The solvents used in common commercial preparations are themselves flammable. While it is possible to work with this compound using cannula transfer, traces of tert-butyllithium at the tip of the needle or cannula may ignite and clog the cannula with lithium salts. While some researchers take this "pilot light" effect as a sign that the product is "fresh" and has not degraded due to time or improper storage/handling, others prefer to enclose the needle tip or cannula in a short glass tube, which is flushed with an inert gas and sealed at each end with septa.[14] Serious laboratory accidents involving tert-butyllithium have occurred. For example, in 2008 a staff research assistant, Sheharbano Sangji, in the lab of Patrick Harran[15] at the University of California, Los Angeles, died after being severely burned by a fire ignited by tert-butyllithium.[16][17][18]
Large-scale reactions may lead to runaway reactions, fires, and explosions when tert-butyllithium is mixed with ethers such as diethyl ether, and tetrahydrofuran. The use of hydrocarbon solvents may be preferred.
See also
[edit]References
[edit]- ^ Bartlett, Paul D.; C. Gardner Swain; Robert B. Woodward (1941). "t-Butyllithium". J. Am. Chem. Soc. 63 (11): 3229–3230. doi:10.1021/ja01856a501.
- ^ a b Elschenbroich, C. (2006). Organometallics. Weinheim: Wiley-VCH. ISBN 978-3-527-29390-2.
- ^ K. P. C. Vollhardt, N. E. Schore (1999). "Organometallic reagents: sources of nucleophilic carbon for alcohol synthesis". Organic Chemistry : Structure And Function, 3rd edition.
- ^ Rick L. Danheiser, David M. Fink, Kazuo Okano, Yeun-Min Tsai, Steven W. Szczepanski (1988). "(1-Oxo-2-Propenyl)Trimethylsilane". Organic Syntheses. 66: 14. doi:10.15227/orgsyn.066.0014.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ John A. Soderquist (1990). "Acetyltrimethylsilane". Organic Syntheses. 68: 25. doi:10.15227/orgsyn.068.0025.
- ^ M. A. Tschantz, L. E. Burgess, A. I. Meyers (1996). "4-Ketoundecanoic Acid". Organic Syntheses. 73: 215. doi:10.15227/orgsyn.073.0215.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Krzysztof Jarowicki, Philip J. Kocienski, Liu Qun (2002). "1,2-Metallate Rearrangement: (Z)-4-(2-Propenyl)-3-Octen-1-Ol". Organic Syntheses. 79: 11. doi:10.15227/orgsyn.079.0011.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Carl A. Busacca, Magnus C. Eriksson, Nizar Haddad, Z. Steve Han, Jon C. Lorenz, Bo Qu, Xingzhong Zeng, Chris H. Senanayake (2013). "Practical Synthesis of Di-tert-Butylphosphinoferrocene". Organic Syntheses. 90: 316. doi:10.15227/orgsyn.090.0316.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Matteson, Donald S.; Majumdar, Debesh (1983). "Homologation of Boronic Esters to α-Chloro Boronic Esters". Organometallics. 2 (11): 1529–1535. doi:10.1021/om50005a008.
- ^ Adam P. Smith, Scott A. Savage, J. Christopher Love, Cassandra L. Fraser (2002). "Synthesis of 4-, 5-, and 6-Methyl-2,2'-bipyridine by a Negishi Cross-Coupling Strategy: 5-Methyl-2,2'-bipyridine". Organic Syntheses. 78: 51. doi:10.15227/orgsyn.078.0051.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mercedes Amat, Sabine Hadida, Swargam Sathyanarayana, Joan Bosch (1997). "Regioselective Synthesis of 3-Substituted Indoles: 3-Ethylindole". Organic Syntheses. 74: 248. doi:10.15227/orgsyn.074.0248.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Stanetty, P; Koller, H.; Mihovilovic, M. (1992). "Directed ortho Lithiation of Phenylcarbamic acid 1,1-Dimethylethyl Ester (N-BOC-aniline). Revision and Improvements". Journal of Organic Chemistry. 57 (25): 6833–6837. doi:10.1021/jo00051a030.
- ^ Fitt, J. J.; Gschwend, H. E. (1984). "Reaction of n-, sec-, and tert-butyllithium with dimethoxyethane (DME): a correction". Journal of Organic Chemistry. 49: 209–210. doi:10.1021/jo00175a056.
- ^ Errington, R. M. (1997). Advanced practical inorganic and metalorganic chemistry (Google Books excerpt). London: Blackie Academic & Professional. pp. 47–48. ISBN 978-0-7514-0225-4.
- ^ "Harran Lab: UCLA". Archived from the original on 2012-10-13. Retrieved 2011-09-21.
- ^ Jyllian Kemsley (2009-01-22). "Researcher Dies After Lab Fire". Chemical & Engineering News.
- ^ Jyllian Kemsley (2009-04-03). "Learning From UCLA: Details of the experiment that led to a researcher's death prompt evaluations of academic safety practices". Chemical & Engineering News.
- ^ Los Angeles Times, 2009-03-01




