Bergman cyclization
| Masamune-Bergman cyclization | |
|---|---|
| Named after | Satoru Masamune
Robert George Bergman |
| Reaction type | Ring forming reaction |
| Identifiers | |
| Organic Chemistry Portal | bergman-cyclization |
| RSC ontology ID | RXNO:0000240 |
The Masamune-Bergman cyclization or Masamune-Bergman reaction or Masamune-Bergman cycloaromatization is an organic reaction and more specifically a rearrangement reaction taking place when an enediyne is heated in presence of a suitable hydrogen donor (Scheme 1).[1][2] It is the most famous and well-studied member of the general class of cycloaromatization reactions.[3] It is named for Japanese-American chemist Satoru Masamune (b. 1928) and American chemist Robert G. Bergman (b. 1942). The reaction product is a derivative of benzene.

The reaction proceeds by a thermal reaction or pyrolysis (above 200 °C) forming a short-lived and very reactive para-benzyne biradical species. It will react with any hydrogen donor such as 1,4-cyclohexadiene which converts to benzene. When quenched by tetrachloromethane the reaction product is a 1,4-dichlorobenzene and with methanol the reaction product is benzyl alcohol.
When the enyne moiety is incorporated into a 10-membered hydrocarbon ring (e.g. cyclodeca-3-ene-1,5-diyne in scheme 2) the reaction, taking advantage of increased ring strain in the reactant, is possible at the much lower temperature of 37 °C.
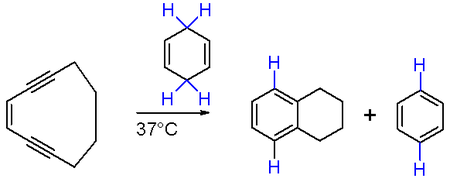
Naturally occurring compounds such as calicheamicin contain the same 10-membered ring and are found to be cytotoxic. These compounds generate the diradical intermediate described above which can cause single and double stranded DNA cuts.[4] There are novel drugs which attempt to make use of this property, including monoclonal antibodies such as mylotarg.[5]
A biradical mechanism is also proposed for the formation of certain biomolecules found in marine sporolides that have a chlorobenzene unit as part of their structure. In this mechanism a halide salt provides the halogen. A model reaction with the enediyene cyclodeca-1,5-diyn-3-ene, lithium bromide as halogen source and acetic acid as hydrogen source in DMSO at 37 °C supports the theory:[6][7]
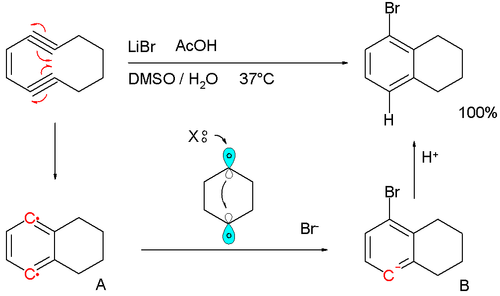
The reaction is found to be first-order in enediyne with the formation of p-benzyne A as the rate-limiting step. The halide ion then donates its two electrons in the formation of a new Br-C bond and radical electron involved is believed to shuttle over a transient C1-C4 bond forming the anion intermediate B. The anion is a powerful base, stripping protons even from DMSO to final product. The dibromide or dihydrogen product (tetralin) never form.

In 2015 IBM scientists demonstrated that a reversible Masamune-Bergman cyclisation of diyne can be induced by a tip of an atomic force microscope (AFM). They also recorded images of individual diyne molecules during this process.[8] When learning about this direct experimental demonstration Bergman commented, "When we first reported this reaction I had no idea that it would be biologically relevant, or that the reaction could someday be visualized at the molecular level.[9]
References
[edit]- ^ Darby, N.; Kim, C. U.; Salaun, J. A.; Shelton, K. W.; Takada, S.; Masamune, S. (1971). "Concerning the 1,5-didehydro[10]annulene system". J. Chem. Soc. D. 1971 (23): 1516–1517. doi:10.1039/C29710001516.
- ^ Jones, Richard R.; Bergman, Robert G. (1972). "p-Benzyne. Generation as an intermediate in a thermal isomerization reaction and trapping evidence for the 1,4-benzenediyl structure". J. Am. Chem. Soc. 94 (2): 660–661. doi:10.1021/ja00757a071.
- ^ Mohamed, R. K.; Peterson, P. W.; Alabugin, I. V. (2013). "Concerted Reactions that Produce Diradicals and Zwitterions: Electronic, Steric, Conformational and Kinetic Control of Cycloaromatization Processes". Chem. Rev. 113 (9): 7089–7129. doi:10.1021/cr4000682. PMID 23600723.
- ^ Lee, May D.; Ellestad, George A.; Borders, Donald B. (1991). "Calicheamicins: discovery, structure, chemistry, and interaction with DNA". Accounts of Chemical Research. 24 (8): 235–243. doi:10.1021/ar00008a003.
- ^ Banfi, Luca; Basso, Andrea; Guanti, Giuseppe; Riva, Renata (2006). "Design and synthesis of heterocycle fused enediyne prodrugs activable at will" (PDF). Arkivoc. HL-1786GR (7): 261–275. doi:10.3998/ark.5550190.0007.719.
- ^ Perrin, Charles L.; Rodgers, Betsy L.; O'Connor, Joseph M. (2007). "Nucleophilic Addition to a p-Benzyne Derived from an Enediyne: A New Mechanism for Halide Incorporation into Biomolecules". J. Am. Chem. Soc. 129 (15): 4795–4799. doi:10.1021/ja070023e. PMID 17378569.
- ^ Borman, Stu (April 2, 2007). "New Route For Halide Addition". Chemical & Engineering News. Retrieved December 30, 2021.
- ^ Schuler, Bruno; Fatayer, Shadi; Mohn, Fabian; Moll, Nikolaj; Pavliček, Niko; Meyer, Gerhard; Peña, Diego; Gross, Leo (2016). "Reversible Bergman cyclization by atomic manipulation". Nature Chemistry. 8 (3): 220–224. Bibcode:2016NatCh...8..220S. doi:10.1038/nchem.2438. PMID 26892552. S2CID 21611919.
- ^ Sciacca, Chris (25 January 2016). "30 Years of Atomic Force Microscopy: IBM Scientists Trigger and Observe Reactions in an Individual Molecule". IBM Research News. IBM. Retrieved 25 January 2016.
External links
[edit]- Bergman Cycloaromatization Powerpoint Whitney M. Erwin 2002
