Bischler–Möhlau indole synthesis
| Bischler-Möhlau indole synthesis | |
|---|---|
| Named after | August Bischler Richard Möhlau |
| Reaction type | Ring forming reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000523 |
The Bischler–Möhlau indole synthesis, also often referred to as the Bischler indole synthesis,[1] is a chemical reaction that forms a 2-aryl-indole from an α-bromo-acetophenone and excess aniline; it is named after August Bischler and Richard Möhlau .[2][3][4][5][6]

Despite its long history, this classical reaction had received relatively little attention in comparison with other methods for indole synthesis, owing to the reactions harsh conditions, poor yields and unpredictable regioselectivity. Recently, milder methods have been developed, including the use of lithium bromide as a catalyst and an improved procedure involving the use of microwave irradiation.[7][8][9]
History
[edit]What is now known as the Bischler-Möhlau indole synthesis was discovered and formulated through the separate, but complimentary, findings of German Scientist Richard Möhlau in 1882[10] and Russia-born German chemist August Bischler (with partner H. Brion) in 1892.[11] These two researchers did not collaborate with each other, but instead independently developed very similar procedures starting from an aromatic ketone structure with an excess of some aniline and ultimately producing a product.[10][11] The images below depict the original indole synthesis equations written by Möhlau and Bischler, respectively:

| Bischler | C6H5COCH2Br + NH3 = C8H7N +HBr + H2O |
|---|---|
| Möhlau | C6H5COCH2Cl + NH3 = C6H5CNCH2 + HCl + H2O |
| (equation notation written as seen in the original articles)[10][11] | |
Being that both scientists had published their works for indole synthesis within the same decade, the general indole synthesis process was given the name Bischler-Möhlau indole synthesis.
This original procedure for the indole synthesis is known to have inconsistent results and yields, but has been modified into new indole synthesis procedures:
- Buu-Hoï[12][1] Modified Indole Synthesis
- Blackhall and Thomson[1][13] Modified Indole Synthesis
- Japp and Murray[1][14] Modified Indole Synthesis
Reaction mechanism
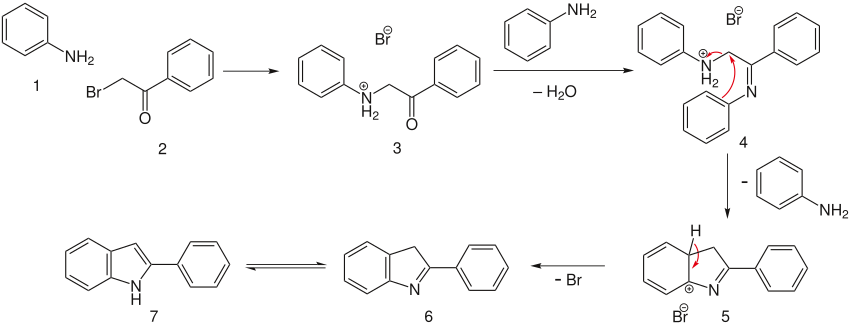
[edit]The first two step involve the reaction of the α-bromo-acetophenone with molecules of aniline to form intermediate 4. The charged aniline forms a decent enough leaving group for an electrophilic cyclization to form intermediate 5, which quickly aromatizes and tautomerizes to give the desired indole 7.[9]
See also
[edit]References
[edit]- ^ a b c d "Bischler Indole Synthesis", Indole Ring Synthesis: From Natural Products to Drug Discovery, John Wiley & Sons, Ltd, 2016-06-17, pp. 249–259, doi:10.1002/9781118695692.ch23, ISBN 978-1-118-69569-2
- ^ Bischler, Aug. (1892). "Ueber die Entstehung einiger substituirter Indole". Berichte der Deutschen Chemischen Gesellschaft. 25 (2): 2860–2879. doi:10.1002/cber.189202502123. ISSN 0365-9496.
- ^ Bischler, Aug.; Fireman, P. (1893). "Zur Kenntniss einiger α-β- Diphenylindole". Berichte der Deutschen Chemischen Gesellschaft. 26 (2): 1336–1349. doi:10.1002/cber.18930260232. ISSN 0365-9496.
- ^ Möhlau, R. (1881). "Ueber die Einwirkung primärer aromatischer Aminbasen auf Acetophenonbromid". Chemische Berichte. 14: 171. doi:10.1002/cber.18810140146.
- ^ Möhlau, R. (1882). "Ueber Diphenyldiisoindol". Chemische Berichte. 15 (2): 2480. doi:10.1002/cber.188201502204.
- ^ Fischer, Emil Hermann; Schmitt, T. (1888). "Ueber Pr-2-Phenylindol". Chemische Berichte. 21: 1071. doi:10.1002/cber.188802101200.
- ^ Pchalek, K.; Jones, A. W.; Wekking, M. M. T.; Black, D. S. C. (2005). "Synthesis of activated 3-substituted indoles: An optimised one-pot procedure". Tetrahedron. 61: 77. doi:10.1016/j.tet.2004.10.060.
- ^ Sridharan, V.; Perumal, S.; Avendaño, C.; Menéndez, J. C. (2006). "Microwave-Assisted, Solvent-Free Bischler Indole Synthesis". Synlett: 91. doi:10.1055/s-2005-922760.
- ^ a b Vara, Yosu; Aldaba, Eneko; Arrieta, Ana; Pizarro, José L.; Arriortua, María I.; Cossío, Fernando P. (2008). "Regiochemistry of the microwave-assisted reaction between aromatic amines and α-bromoketones to yield substituted 1H-indoles". Organic & Biomolecular Chemistry. 6 (10): 1763–72. doi:10.1039/B719641E. PMID 18452011.
- ^ a b c d Möhlau, Richard (1881-01-01). "Ueber die Einwirkung primärer aromatischer Aminbasen auf Acetophenonbromid". Berichte der Deutschen Chemischen Gesellschaft. 14 (1): 171–175. doi:10.1002/cber.18810140146. ISSN 0365-9496.
- ^ a b c d Bischler, Aug. (1892-07-01). "Ueber die Entstehung einiger substituirter Indole". Berichte der Deutschen Chemischen Gesellschaft. 25 (2): 2860–2879. doi:10.1002/cber.189202502123. ISSN 0365-9496.
- ^ Buu-Hoï, Ng. Ph.; Jacquignon, P.; Loc, T. B. (1958). "143. Carcinogenic nitrogen compounds. Part XXIV. The synthesis of indole and quinoline compounds from cyclic ketones". J. Chem. Soc.: 738–740. doi:10.1039/jr9580000738. ISSN 0368-1769.
- ^ Blackhall, A.; Thomson, R. H. (1954). "Aromatic keto-enols. Part III. Some heterocyclic quinols". Journal of the Chemical Society (Resumed): 3916. doi:10.1039/jr9540003916. ISSN 0368-1769.
- ^ Japp, Francis R.; Murray, T. S. (1894). "LXXII.—Preparation of 2′ : 3′-diphenylindoles from benzoïn and primary benzenoid amines". J. Chem. Soc., Trans. 65: 889–899. doi:10.1039/ct8946500889. ISSN 0368-1645.

